COVID-19
Jan 06, 2022
Siemens Healthineers’s COVID-19 Antigen Self-Test; Paragon’s R3ACT Stabilization System; Pulnovo Medical’s PADN-CFDA trial; Endotronix’s PROACTIVE-HF trial; Abbott’s Proclaim XR SCS System; RTI Surgical spins off businesses
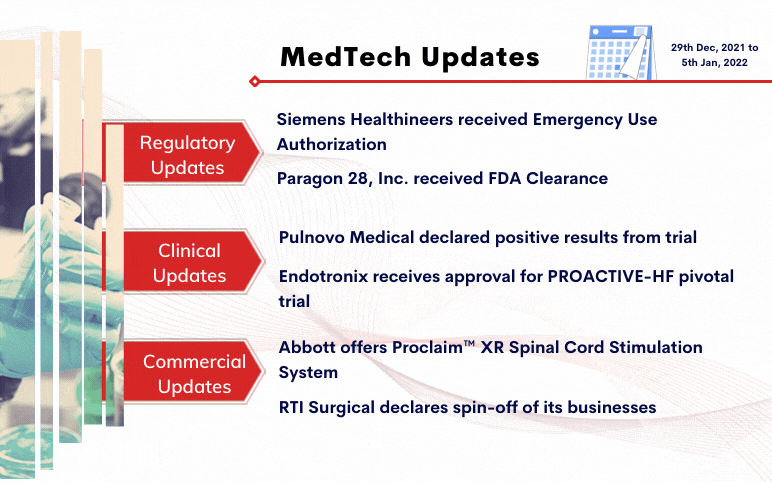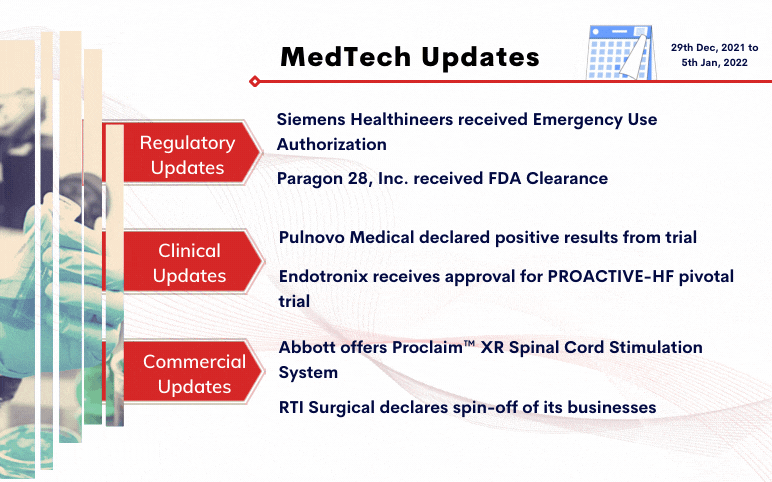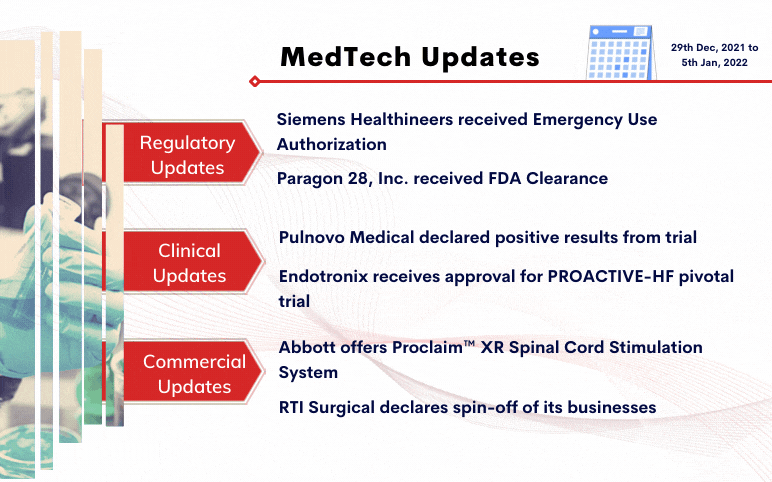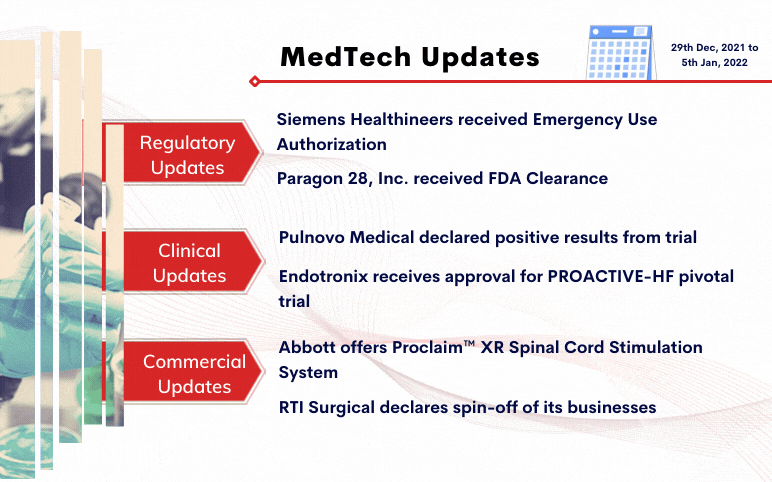
Siemens Healthineers received Emergency Use Authorization for CLINITEST® Rapid COVID-19 Antigen Self-Test by the FDA On December 29, 2021, Siemens Healthineers was granted Emergency Use Authorization (EUA) for its CLINITEST Rapid COVID-19 Antigen Self-Test by the United States Food and Drug Administration ...
Read More...
Jan 04, 2022
Accutar’s Phase I clinical trial for AC0176; Bio-Thera’s cancer drug, BAT6005; Nykode’s Covid-19 vaccine trial; Heat Biologics acquires Elusys
FDA approves IND application of Accutar for a prostate cancer treatment trial The US Food and Drug Administration (FDA) has granted Accutar Biotechnology’s Investigational New Drug (IND) application for Phase I clinical trial of AC0176 for treating metastatic castration-resistant prostate cancer (mCRPC). An i...
Read More...
Jan 03, 2022
What are Monoclonal Antibodies and How Can They Treat COVID-19?
An antibody is a protein produced by the immune system in response to an infection in the body. Whereas, a monoclonal antibody is a molecule produced in a laboratory specifically designed to enhance the body’s natural immune system response against an invader, such as cancer or an infection. Monoclonal antibodies h...
Read More...
Dec 30, 2021
Cerner, Scanwell Health and Personal Genome Diagnostics Acquisition; Zynex’s Fluid Monitoring System, CM-1500; Sight Sciences’ TearCare System; Envista’s Nobel Biocare N1 Implant System
Oracle Corporation sets to acquire Cerner Corporation On December 20, 2021, Oracle Corporation and Cerner Corporation entered into an agreement as per which Oracle will acquire Cerner through an all-cash tender offer for approximately USD 28.3 billion in equity value. Larry Ellison, Chairman and C...
Read More...
Dec 28, 2021
Biogen-Eisai’s Aduhelm; Quidel acquires Ortho Clinical Diagnostics; Accutar Biotechnology’s AC0176; Boehringer’s Spesolimab
Biogen-Eisai's potential Aduhelm sequel drug secured speedy review by FDA Biogen and Eisai’s Alzheimer’s disease treatment-in-waiting, lecanemab, has been secured a fast-track tag by the FDA, setting up a potentially swift path through the regulatory process. The drug is the next in line behind the pair’s app...
Read More...
Dec 10, 2021
Contract Organizations Impacting Pharma Development and Manufacturing
Contract Development and Manufacturing Organisation (CDMO) is a term for organizations providing extensive services and solutions to the pharmaceutical and biotech industry starting from the drug development process to packaging of the product. These companies deliver a broad range of services, such as clinical res...
Read More...
Dec 09, 2021
Boston Scientific’s MODULAR CRM System; Restore Medical’s ContraBand clinical trial result; BD acquires Venclose; Roche differentiates mutations in Omicron; Linus’s StrandDx-ASD; HelioLiver launches liquid biopsy test
Boston Scientific initiates trial to evaluate industry's first Modular CRM System On December 02, 2021, Boston Scientific Corporation started the MODULAR ATP clinical trial to examine the safety, performance, and effectiveness of the mCRM™ Modular Therapy System. The system comprises of two cardiac ...
Read More...
Dec 02, 2021
LensGen’s Juvene Intraocular Lens; Nevro announces clinical data publications; FDA Clearance to Immunexpress’s SeptiCyte; GE unveils AI and Digital Technologies; Canon launches PIQE DLR and SilverBeam; Hyperfine’s Swoop gets FDA clearance
Canon Medical launches super-resolution deep-learning reconstruction for Cardiac CT scans On November 28, 2021, Canon Medical launched the Precise IQ Engine (PIQE) DLR and SilverBeam filter in the recent version of the Aquilion ONE / PRISM edition. PIQE, super-resolution deep-learning reconstruction techno...
Read More...
Aug 31, 2021
Ascendis’s SKYTROFA; MicroTransponder’s VNS System (Vivistim); BioMarin’s Voxzogo; Zimmer-Canary’s Smart Knee Implant; COVID-19 impact on Solid Organ Transplants
FDA's Green Flag to Ascendis's Once-Weekly SKYTROFA for Treatment of Pediatric Growth Hormone Deficiency For the treatment of growth failure due to deficiency of growth hormones, the USFDA approved Ascendis Pharma's Skytrofa (lonapegsomatropin-tcgd) for children of one year and older suffering from an improper s...
Read More...
Jul 07, 2021
Charting out the COVID-19 Vaccine Landscape
The uncontrolled outbreaks of Covid-19 have brought unprecedented challenges to the world community. It has created an imbalance in mental, social, and economic well-being. Apart from this, it has changed the overall way of living and has created fear and anxiety among people. Countries are experiencing peaks, one ...
Read More...





-Agonist.png)

