Diabetes
Nov 16, 2022
Insights Into the Key Advancements Transforming the Type 2 Diabetes Treatment and Management
Diabetes is a chronic metabolic disorder that is influenced by a wide variety of factors and is characterized by high or low blood glucose (or blood sugar) levels in the body. Diabetes is a major risk factor for blindness, kidney failure, heart attacks, stroke, and lower limb amputation. Also, it can significantly ...
Read More...
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...
Jul 19, 2022
Byondis’s HER2-targeting ADC trastuzumab duocarmazine; AbbVie Migraine Drug Atogepant; Grünenthal Acquires Bayer’s Testosterone Drug Rights; Vertex Acquires ViaCyte; Merck & Orion Announces Collaboration; Verve Starts Trials of Cholesterol Drug; Kyowa Kirin Drops Nourianz follow-up KW-6356; FDA Orphan Drug and Fast Track Designations to CV-01
Byondis Files its HER2-Targeting Antibody-Drug Conjugate (ADC) Trastuzumab Duocarmazine in the US and Europe Byondis has filed for clearance of its HER2-targeting antibody-drug conjugate (ADC) trastuzumab duocarmazine in the United States and Europe, setting up a battle with heavyweight competitors Roche and Ast...
Read More...
Jun 23, 2022
CE Mark to Ibex’s Gastric Cancer Detection System; Senseonics’s Eversense E3 Continuous Glucose Monitoring System; NEUSPERA’s NUVELLA SYSTEM; Conformal Medical Initiates CONFORM Pivotal Trial; Meridian Launches New qPCR Master Mixes for Stool Samples; Sentinel Diagnostics Launches SENTiFIT 800
Conformal Medical Announces Launch of CONFORM Pivotal Trial On June 17, 2022, Conformal Medical Inc, is a medical device company manufacturing devices to avoid strokes in patients with non-valvular atrial fibrillation and developing next-generation LAAO technology. Its exclusive technology is intended to make le...
Read More...
Jun 09, 2022
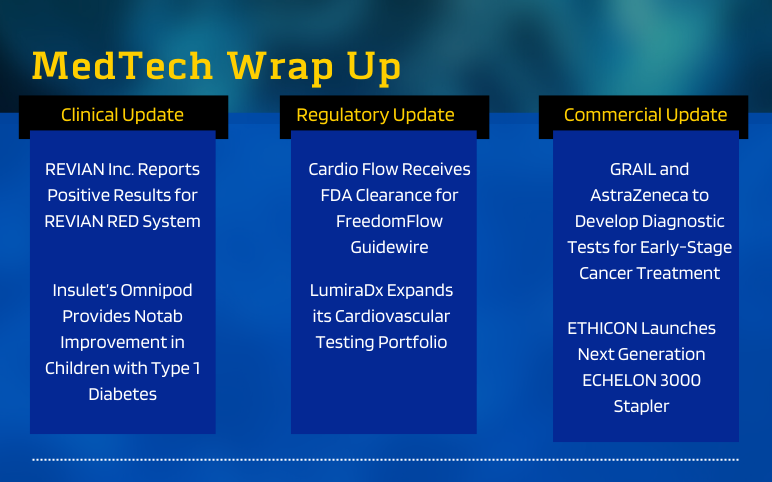
REVIAN RED System Delivers Positive Results, Insulet’s Omnipod Provides Notable Improvement in Type 1 Diabetes, FDA Clearance to Cardio Flow’s FreedomFlow Guidewire; LumiraDx’s Cardiovascular Testing Portfolio; GRAIL-AstraZeneca to Develop Diagnostic Tests; ETHICON Launches Next Generation ECHELON 3000 Stapler
Positive Results Report REVIAN RED System as a Possible Treatment for Central Centrifugal Cicatricical Alopeciain African American Women On June 02, 2022, REVIAN Inc., an aesthetic medical technology company focused on stimulating the body's natural processes in order to rejuvenate hair and skin with light relea...
Read More...
Jun 03, 2022
Diabetic Retinopathy: The Hornet’s Nest of Diabetes Mellitus
Diabetic Retinopathy (DR), a microvascular disease, is an eye condition in diabetic patients, affecting blood vessels in the retina and causing significant visual loss in working populations. The retina needs a constant supply of blood, which it receives through a network of tiny blood vessels. Constant high blood ...
Read More...
May 12, 2022
Abbott’s Alinity m STI Assay; Phillips’ MR 7700 System; HOYA’s MiYOSMART Spectacle Lens; Magneto’s Pulmonary Embolism Treatment; Owen Mumford and Stevanto Group’s Collaboration Deal; Elekta’s Radiosurgery System-Elekta Esprit
Abbott Receives Regulatory Approval from US FDA for Alinity™ m STI Assay On May 04, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Alinity™ m STI Assay developed by Abbott which is capable of simultaneously detecting and differentiating four common sexually trans...
Read More...
Apr 28, 2022
MEDIT’s i700 Intraoral Scanner; Phantom Neuro and Blackrock’s Next-Generation Assistive Devices; Fresenius Medical’s Versi PD Cycler System; Franklin Mountain’s Dual Lumen Catheter for Cardiac Applications; Bioretec’s Bioresorbable Implant; Vectorious’s VECTOR-HF II Study
MEDIT Announces Launch of i700 Intraoral Scanner On April 20, 2022, based on the success of i700 intraoral scanner, MEDIT Corp launched the all new i700 wireless intraoral scanner is light, fast, accurate, and now – wire-free. i700 wireless is a lightweight, wire-free device that offers a smooth and qui...
Read More...
Apr 25, 2022
Rise In Bispecific Antibodies Utilization As Antibody Therapeutics
Bispecific Antibodies (BsAbs) are antibodies that contain two different antigens or have two different epitopes on the same antigen. It is observed for Bispecific Antibodies in clinical trials that the therapeutic effects of these are considerably higher than those of monoclonal antibodies (MoAbs). There are a vast...
Read More...
Mar 31, 2022
Ypsomed-CamDiab’s Collaboration; Vela Diagnostics’s NGS-Based Pan-Cancer Panels; Fresenius’s SMOFlipid Lipid Injectable Emulsion; Sientra Gained Regulatory Nod to Market Breast Implants; Synchron’s Endovascular BCI Stentrode Device; HLT’s TAVR Clinical Studies
Ypsomed Collaborated with CamDiab for Smartphone-based Automated Insulin Delivery On March 22, 2022, Ypsomed, a leading manufacturer of injection and infusion systems for self-medication partnered with CamDiab Ltd, developer of CamAPS FX, an app for the management of glucose levels via insulin pumps using an ada...
Read More...








-Agonist.png)

