Rise In Bispecific Antibodies Utilization As Antibody Therapeutics
Apr 25, 2022
Table of Contents
Bispecific Antibodies (BsAbs) are antibodies that contain two different antigens or have two different epitopes on the same antigen. It is observed for Bispecific Antibodies in clinical trials that the therapeutic effects of these are considerably higher than those of monoclonal antibodies (MoAbs). There are a vast number of applications of Bispecific Antibodies that can be incorporated into tumor immunotherapy and also for the treatment of many other diseases. Recently, with progress in antibody or protein engineering and novel recombinant DNA technology, various platforms for generating different types of Bispecific Antibodies based on novel strategies, for various uses, have been established. More than 30 mature commercial technology platforms have been used to create and develop Bispecific Antibodies based on the heterologous recombination of heavy chains and matching of light chains.
The detailed mechanisms of clinical/therapeutic action have been demonstrated with these different Bispecific Antibodies types. Many Bispecific Antibodies types have received market approval, and there are more than 110+ Bispecific Antibodies in clinical trials at various stages. Bispecific Antibodies have already shown great therapeutic potential in cancer and other diseases, such as diabetes, Alzheimer’s disease and other ophthalmological diseases.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Biohaven’s Troriluzole Failure; Daiichi/ AZ’s Enhertu; Fujifilm & Manufacturing ...
- Eli Lilly’s ZEPBOUND Surpasses WEGOVY in SURMOUNT-5 Trial; Verastem Oncology Secures FDA Approval...
- Snippet
- Future Avenues for Prediabetes Treatment: The Road Ahead
- Bayer’s CKD Drug; Enochian’s RNA Hijack for HBV; Novo Nordisk’s to get Prothena’...
Bispecific Antibody Structure
Bispecific Antibodies are present in many formats, but main types of Bispecific Antibodies include IgG-like and non-IgG-like. Main manufacturing methods include quadromas, genetic recombination, chemical conjugation, and each method results in a unique format. On the basis of their different properties, Bispecific Antibodies are classified into two distinct types: IgG-like Bispecific Antibodies and non-IgG-like Bispecific Antibodies, and their detailed structure is as follows-
IgG-like Bispecific Antibodies
IgG-like format constitutes the traditional monoclonal antibody (mAb) structure of two Fab arms (fragment antigen-binding) and one Fc region (fragment crystallization), except the two Fab sites bind different antigens. The most common formats are called trifunctional antibodies, also they have three unique binding sites on the antibody: the two Fab regions, and the Fc region. Each light and heavy chain pair is from a unique mAb. The Fc region made from the two heavy chains forms the third binding site. These Bispecific Antibodies are often manufactured with the quadroma, or the hybrid hybridoma, method. However, the quadroma method relies on random chance to form usable Bispecific Antibodies, and can be inefficient. Another method for manufacturing IgG-like Bispecific Antibodies is called “knobs into holes,” and relies on introducing a mutation for a large amino acid in the heavy chain from one mAb, and a mutation for a small amino acid in the other mAb’s heavy chain. This allows the target heavy chains (and their corresponding light chains) to fit together better, and makes the production of Bispecific Antibodies more reliable.
Non-IgG-like Bispecific Antibodies
There are other Bispecific Antibodies that lack an Fc region entirely, leading to relatively simple design strategies. These include chemically linked Fabs, consisting of only the Fab regions, and various types of bivalent and trivalent single-chain variable fragments (ScFvs). Proper utilization of engineered peptide linkers, scFvs can form dimers, trimers, tetramers, pentamers and even higher order oligomers. The bispecific T-cell engager (BiTE) is one type of tandem scFvs that constitutes of two scFvs, one binds to CD3 on T cells and the other one binds to a surface antigen on tumor cells to redirect T cells to kill tumor cells. Using this approach, Blinatumomab (Blincyto) has been FDA approved for Acute Lymphoblastic Leukemia (ALL) treatment. There are also fusion proteins mimicking the variable domains of two antibodies.
Design and Engineering of Bispecific Antibodies
In the beginning, Bispecific Antibodies were developed using the quadroma method (hybrid hybridoma). Because of the random assembly of two different heavy and two different light chains, only one functional Bispecific Antibody existed whereas the other nine variants are either non-functional or monospecific. This leads to a low yield of target Bispecific Antibodies which poses a big challenge to the downstream purification process. To overcome the heavy and light chain association issue, scientists have focused on recombinant DNA technology to engineer Bispecific Antibodies.
Mechanisms of Action of Bispecific Antibodies
Bispecific Antibodies have two binding sites located at different antigens for recognition of two different epitopes of one antigen simultaneously, the functioning pathways are quite flexible. Some Bispecific Antibodies play the role of immune cell connector, connecting tumor cells to immune cells. Most of these Bispecific Antibodies target CD3, CD47, and CD16, also some release immune cells from an immunosuppressed state for dual-targeted immune checkpoints, such as LAG-3, PD1, CTLA-4. Other Bispecific Antibodies target the immune checkpoint and TAAs, while other Bispecific Antibodies target CD28 and CD137 to activate immune cell activity. On top of targeting immune cells, Bispecific Antibodies target tumor antigens, blocking dual signaling pathways. The main targets are PSMA, HER2, HER3, EGFR, DLL1, and ANG-2. Besides targeting dual tumor targets, some target inflammatory factors in the tumor microenvironment to reduce inflammation and CRS. Most Bispecific Antibodies target tumors, although some are used for hemophilia A treatment, neovascular age-related macular degeneration, diabetes, pneumonia caused by bacteria, and many more.
Applications of Bispecific Antibodies
Bispecific Antibodies are massively used in both the diagnosis and therapy of several diseases.In respect to diagnosis, Bispecific Antibodies can even be combined with HRPO; BsAbs can be used in pre-targeting strategies to assist in clinical diagnosis; and provide better imaging for the early detection, diagnosis, and treatment of cancer. On the other hand, in terms of therapy, the main strategy is to precisely target and reactivate immune cells, help regulate the activation of immune cells, fine-tune the fate and function of immune cells, improve the tolerance of immune cells, and promote a return to immune homeostasis. In addition to tumors, Bispecific Antibodies can treat other diseases.
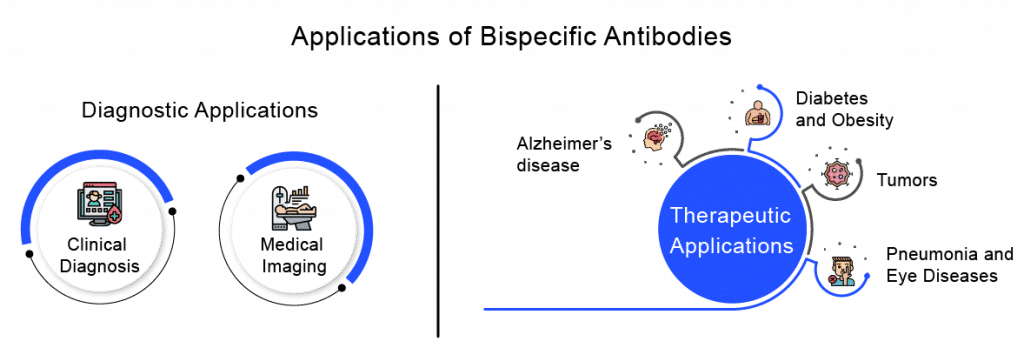
Diagnostic Applications
Clinical Diagnosis
Because Bispecific Antibodies can bind to specific detection sites and specific antigens simultaneously, their design is sensitive, flexible, and convenient. They can reduce the harmful effects of chemical modification of enzymes or antibodies, simplify detection, and help detect infectious bacterial and viral diseases and diagnose cancer. Lipoarabinomannan (LAM), is considered to be an important non-protein antigen of the cell wall of tuberculosis bacteria, and it is found in various bodily fluids of infected patients. Bispecific Antibodies that target LAM and HRPO can be used for the rapid detection of tuberculosis bacteria. Staphylococcus aureus is the main cause for wound infection. Bispecific Antibodies target HRPO and S. aureus thermal nuclease (TNase) for enabling the detection of the presence of S. aureus. Finally, Bispecific Antibodies targeting of the nuclear plasmid protein of the SARS coronavirus immunoassay antigen is also considered to be superior to that by MoAb in terms of sensitivity, specificity, and signal strength while maintaining a minimal signal background.
Medical Imaging
18F fluorodeoxyglucose positron emission tomography (FDG-PET) is able to identify cancer and new potential lesions in clinical diagnosis. However, FDG-PET has some limited sensitivity to tumors smaller than 1 cm and also lacks specificity for inflammatory or infectious lesions. To combat those, pre-targeted detection based on Bispecific Antibodies is used in clinics. First, a Bispecific Antibody that specifically recognizes both tumor antigens and small molecule polypeptides is injected into the patient. Then, a small radiolabeled molecule is injected into the tumor location and combined with the pre-targeted Bispecific Antibody when the concentration of BsAb in the blood increases to achieve blood clearance. The tumor is assessed based on radioactive radiation, and the excess radiolabeled small molecule is excreted. This method has high binding specificity, sensitivity, and safety.
Therapeutic Applications
Tumors
It is observed that almost over 86% of the Bispecific Antibodies developed till date are used in cancer treatment. During the targeted cancer therapy, the half-life of Bispecific Antibodies can be changed with their molecular size. For some other BsAbs of small molecular size, such as those on the BiTE platform, it is necessary to increase their half-life, for example by adding a section of Fc to avoid multiple infusions. Some nanobodies extend the half-life by conjugation. For example, adding a human serum albumin binding domain can enlarge the molecular size and promote the localization of Bispecific Antibodies.
Alzheimer’s Disease
Amyloid β protein (Aβ) has been associated many times with Alzheimer’s disease (AD) as it constitutes a major component of the extracellular plaque found in AD brains. A Bispecific Antibody namely BI 1034020 aims to target two different epitopes of Aβ (Aβ40 and Aβ42) with high affinity. Its goal is to reduce the level of free Aβ peptide in plasma and thus prevent the formation of new Aβ plaques and clear existing plaques.
In terms of crossing the blood-brain barrier (BBB), ANG4043 – a BsAb is evident in providing new strategies to target Angiopep-2 (An2) and HER2. An2 can bind to low-density lipoprotein-like receptor 1 (LRP1), which effectively crosses the BBB through receptor-mediated endocytosis and is taken up by cells expressing LRP1. Preclinical evidence on ANG4043 shows that it not only treats HER2-positive brain metastasis but is also helpful in treating various brain tumors and other diseases of the central nervous system.
Diabetes and Obesity
FGFR1 (fibroblast growth factor receptor 1) improves insulin sensitivity, accelerates weight loss, and ameliorates liver steatosis. RG7992 (BFKB8488A) is a Bispecific Antibody that targets this FGFR1 and the coreceptor β klotho (KLB) as well. Even though FGFR1 is highly expressed in many cells, β-klotho is only restricted to the liver and adipose tissues. Therefore, the BsAb – RG7992 can avoid extensive FGFR activation and provide clinical benefits for patients dealing with Diabetes and Obesity.
Pneumonia and Eye Diseases
The overuse of antibiotics has led to a sudden increase in bacterial resistance, and Pseudomonas aeruginosa has turned out to be one of the main culprits behind clinical lung infections. In one of the experimental rabbit lung infection models, MEDI3902 which is a Bispecific Antibody targeting the type 3 secreted protein (PcrV) and Psl exopolysaccharide has a protective effect in treatment and prevention of lung infection. In animals treated with MEDI3902, Pseudomonas aeruginosa was found to be greatly reduced, and the organ burden and the expression of pro-inflammatory mediators in lung tissue were also observed to be decreased significantly.
Neovascular age-related macular degeneration (nAMD) is considered to be the main cause of vision loss in the elderly people. The current standard of care for nAMD treatment is to inhibit Vascular endothelial growth factor (VEGF). ANG-2 is a growth factor that plays a key role in angiogenesis, vascular homeostasis, and vascular permeability. Faricimab (RG7716) is a BsAb targeting VEGF-A and ANG-2 shows greater efficacy than anti-VEGF-A alone in a laser-induced non-human primate model.
List of Bispecific Antibody Drugs
Contrary to Monospecific Antibodies, Bispecific Antibodies are antibodies containing two antigen-binding sites and therefore can simultaneously target two different epitopes. In January 2022, Faricimab (Vabysmo, Roche) was FDA approved for Wet Age-related Macular Degeneration (AMD) treatment and Diabetic Macular Edema (DME) treatment. Besides the first Bispecific Antibodies Catumaxomab being withdrawn in 2017, to this date, this is the fourth Bispecific Antibody drug on the market now. With the increased attention in the antibody field, Bispecific Antibodies have accounted for nearly 20% of the clinical antibody pipeline, with about 150+ Bispecific Antibodies currently in clinical trials. Major Bispecific Antibodies producing companies include drug giants such as Genmab, Roche, Amgen, Trion whereas companies having BsAbs in the pipeline include Biointron, Akeso Inc, MacroGenics Inc, Xencor Inc, Alphamab Oncology, Johnson & Johnson, Zymeworks Inc, Regeneron Pharmaceuticals, University of Texas MD Anderson Cancer Center, and many more.

Way Ahead For Bispecific Antibodies
The recent and rapid development of recombinant DNA technology, also with the deep understanding of antibody engineering, diversity in Bispecific Antibody formats can be observed. These Bispecific Antibodies are under several clinical trials in the emerging pipeline to pursue optimal biological activity and clinical purposes. Bispecific Antibodies have demonstrated good therapeutic potential in oncology and other diseases such as diabetes, ophthalmological disease, and Alzheimer’s disease. The future BsAbs development can witness the involvement of developing and using new targets, new platforms, new combinations as well as new geometric configurations, and on top of that combined treatments with traditional biological drugs, other forms of immunotherapy, physical and chemical therapies. Bispecific Antibodies also have the potential to become the new focus of future medical and biological research and development. Currently, numerous Bispecific Antibodies are entering several classes of clinical development and it can be estimated that there will be more Bispecific Antibodies getting marketing approval in the future.
Downloads
Article in PDF
Recent Articles
- Alnylam’s HELIOS-B Phase III Study of Vutrisiran; Bristol Myers Squibb Secures FDA Nod for ...
- A New Era for Multiple Myeloma Treatment: Bispecific Antibodies Enter the Fray
- World Diabetes Day | 2019
- 8 Emerging Obesity Trends Transforming Therapeutics Segment
- New Approaches and Evolutions to Reshape the Diabetes Management and Care