Hepatitis B market
Sep 16, 2025
Lion TCR Secures Triple FDA Milestones with IND Clearance for Chronic Hepatitis B; Corstasis Therapeutics Wins FDA Approval for ENBUMYST Nasal Spray to Treat Edema in CHF, Liver, and Kidney Disease; Amneal Gains FDA Approval for Sodium Oxybate Oral Solution in Narcolepsy Patients with Cataplexy and Excessive Daytime Sleepiness; Greenwich LifeSciences’ GLSI-100 Granted FDA Fast Track Designation; Akeso’s Ligufalimab Granted FDA Orphan Drug Designation for Acute Myeloid Leukemia
Lion TCR Secures Triple FDA Milestones for Chronic Hepatitis B with IND Clearance of TCR-T Therapy Lion TCR announced that the FDA has cleared its Investigational New Drug (IND) application for LioCyx-M004, authorizing the initiation of phase Ib/II clinical trials in patients with chronic hepatitis B (CHB). The ...
Read More...
Jul 29, 2019
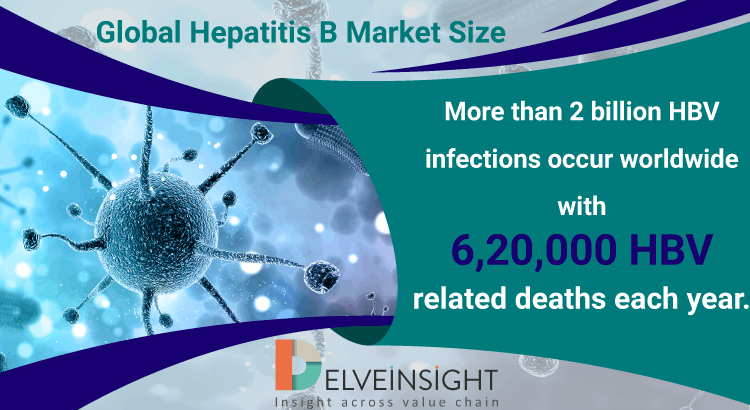
Global Hepatitis B Market Size
1 in every 100,000 persons get infected with Hepatitis B virus every year at a stable rate from the past three decades. Moreover, the WHO estimated that there are more than 2 billion HBV infected people and about 378 million chronic Hepatitis B virus carriers worldwide. Hepatitis B, often life-threatening, is a...
Read More...
Jul 28, 2019
World Hepatitis Day
World Hepatitis Day is observed on 28 July of every year, aims to bring the world together to raise the awareness of the global burden of viral hepatitis and bring real change. Hepatitis B virus is a blood-borne and transmitted sexually by the virus, which is acquired by percutaneous and exposure to blood or ot...
Read More...


-Agonist.png)

