Keytruda
Dec 02, 2022
PD-1 and PD-L1 Inhibitors: Will They Dominate Over Conventional Cancer Therapies?
The development of tumor therapy in cancer immunotherapy has been aided by programmed death receptor-1 and programmed death protein ligand inhibitors. Since its discovery in 1990, the programmed cell death protein 1 receptor (PD-1) receptor has been known to negatively regulate T-cell mediated immune response via i...
Read More...
May 31, 2022
Bristol-Myers Squibb’s Opdivo Approval; Mirati’s KRAS-inhibitor Adagrasib; J&J and Legend Biotech’s CAR-T Carvykti Approval; Roche’s Glofitamab; Arena Pharma’s Etrasimod Phase 3 Trials; FDA Approves Dermavant’s Vtama; FDA Approves Novartis’ Cell Therapy; NICE Approves Merck’s Keytruda
Bristol-Myers Squibb’s Opdivo Gets FDA Approval for Esophageal Cancer The FDA has approved two combination drug regimens based on Bristol-Myers Squibb’s PD-1 inhibitor Opdivo for previously untreated advanced Esophageal Cancer, encroaching on the territory belonging to Merck & Co’s rival Keytruda. The latest...
Read More...
Jan 10, 2022
Novel and Emerging Metastatic Colorectal Cancer Treatment Drugs Anticipated to Change Market Dynamics
Colorectal cancer is a disease in which malignant (cancer) cells form in the tissues of the colon or the rectum. Colorectal Cancer is the third most lethal and fourth most commonly diagnosed cancer in the world. In 2020, nearly 2 million new cases were expected, with approximately 1 million deaths across the globe....
Read More...
Jun 05, 2020
Prostate Cancer Market Experiences an Influx of the Pharma Players Veering the Market Ahead
Prostate Cancer Market Size is Expected to Increase with a CAGR of 8.1% in the 7MM for the study period of 2017-30 owing to rich Pipeline, Increasing Awareness and Better Treatment Options. A malignancy of prostate glands, Prostate Cancer, develops slowly and remains confined to prostate glands, however, can inv...
Read More...
Jan 15, 2020
Malignant Pleural Mesothelioma Upcoming Therapies
The Malignant Pleural Mesothelioma Market includes several immunotherapy agents, in the pipeline including Keytruda, Opdivo and Yervoy, which have shown promising anticancer activity against mesothelioma in recent clinical trials. These drugs have been approved for other indications and now are being used in mono...
Read More...
May 23, 2019
The Business Cocktail
Merck decides to acquire Peloton Therapeutics, fortifying Oncology pipeline Merck is ready to buy Peloton Therapeutics for USD 1.1 million. The companies have entered into a definitive agreement which will give Merck rights over an oral HIF-2α inhibitor- PT2977, which is Peloton’s lead candidate for the treatme...
Read More...
May 02, 2019
The Business Cocktail
EISAI now solely responsible for lemborexant Eisai today announced that its U.S. subsidiary has bought out Purdue Pharma L.P.’s rights in the worldwide collaboration for the development and commercialization of lemborexant, an investigational sleep-wake regulation agent being studied for the treatment of multip...
Read More...
Apr 23, 2019
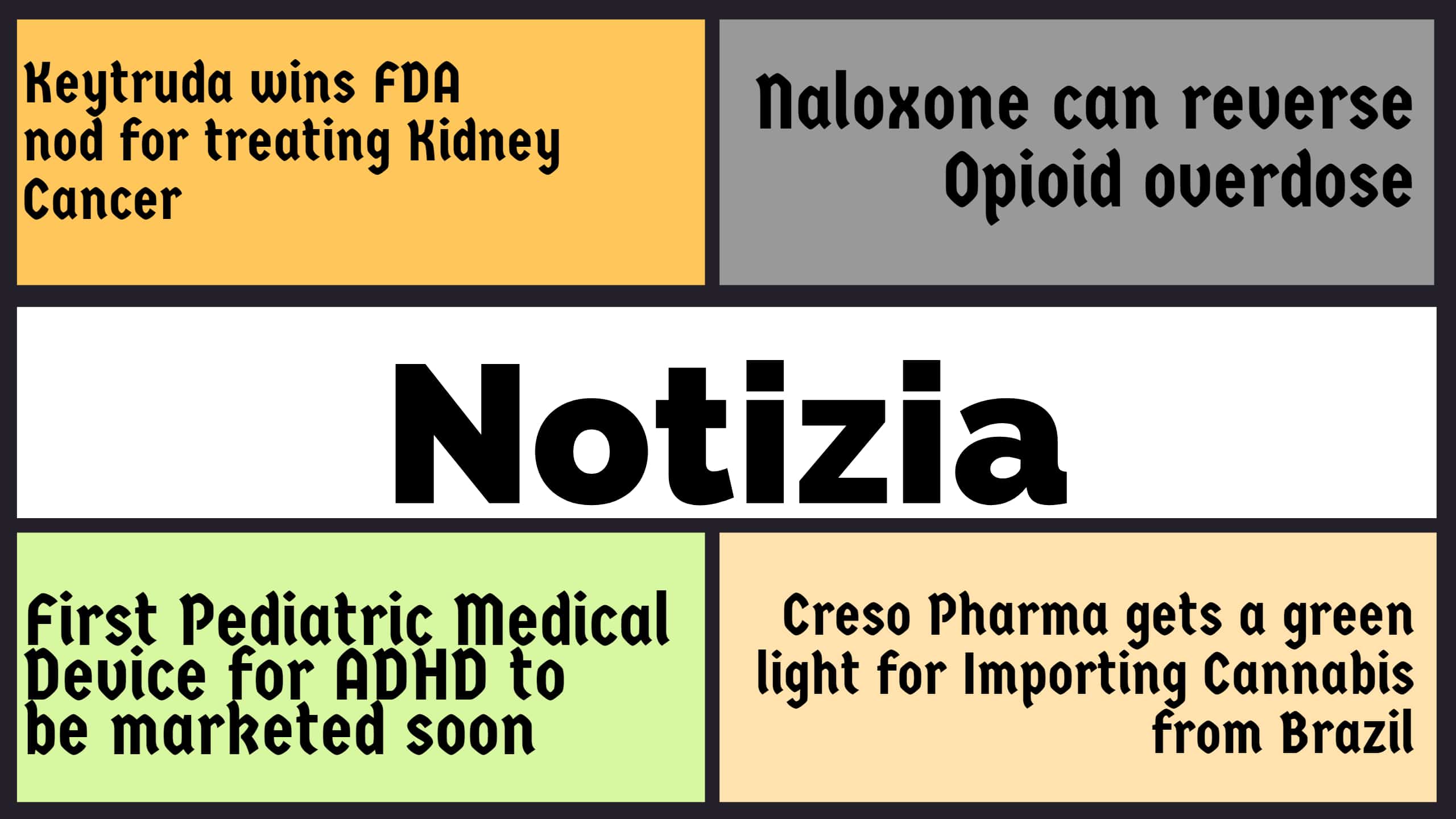
Notizia
Keytruda wins FDA nod for treating Kidney Cancer Merck’s Keytruda has recently got the U.S. FDA approval to be administered for Kidney Cancer. The drug Keytruda was approved in combination with Pfizer’s Inlyta for the treatment of advanced renal carcinoma. This therapy could prove to be serious for competition B...
Read More...
Nov 12, 2018
Virus, the Cancer Therapy of the Future
Could a virus be a treatment therapy? Confusing, isn't it? On the face value, it seems no; but on delving deeper, the confusion dissipates. There have been tireless efforts among a section of scientists to convert certain viruses through biotechnology techniques into anti-disease agents to infect and destroy cancer ...
Read More...
Mar 22, 2018
Business Cocktail
Merck and Eisai has entered into an Oncology Pact worth Up to USD 5.76 Billion Two major pharmaceutical companies (Merck & Eisai) has entered into a USD 5.76 Billion pact to develop and commercialize Lenvima (lenvatinib mesylate) both as a monotherapy and an in-combination therapy for treating multiple types of ...
Read More...







-Agonist.png)

