Roche
Oct 30, 2018
Notizia
TherapeuticsMD bags FDA approval for hormone therapy The FDA has nodded hormone therapy of TherapeuticsMD for hot flashes that are associated with menopause. Bijuva is an oral treatment that contains molecularly identical (bio-identical) versions of the hormones estradiol and progesterone for menopausal women with a...
Read More...
Sep 27, 2018
Ceribell raises $35M; Lyra reels $29.5M; Minoryx raises Euro21M; Alexion acquired Syntimmune
Ceribell raises USD 35 Million in series B round Ceribell, Emergency EEG maker raised USD 35 million for commercializing its EEG system that can rapidly diagnose patients, who are supposed of not having convulsive seizures with no visual symptoms for the use in neurological intensive care units and emergency rooms. ...
Read More...
Sep 06, 2018
4D Molecular Therapeutics raises; Arvinas slates for IPO; Sutro ruminates for $ 75M; Emergent BioSolutions remunerates Narcan maker
Gene therapy company 4D Molecular Therapeutics raises USD 90 Million to collaborate with AstraZeneca and Roche for moving gene therapies into clinical testing 4D Molecular Therapeutics has upraised USD 90 million in venture capital funds for progressing its vector platform and treatments, which use adeno-associate...
Read More...
Aug 31, 2018
AbbVie Receives Approval; Kymriah Approved; Shire Gets USFDA Approval; NICE Rejects; Bristol-Myers Squibb’s Opdivo; Eisai and Merck Get USFDA Approval
AbbVie Receives USFDA Approval for Imbruvica Plus Rituximab as First Chemotherapy-Free Combination Treatment for Waldenström's Macroglobulinemia Adults AbbVie announced that the USFDA approved Imbruvica (ibrutinib) plus rituximab (Rituxan) for the treatment of adult patients with Waldenström's macroglobulinemia (W...
Read More...
Feb 22, 2018
The Business Cocktail : Latest Pharma deals
Ionis pharmaceuticals recently licensed-out its antisense drug to AstraZeneca in deal total worth up to USD 330 million Ionis Pharmaceuticals has recently licensed-out an antisense drug to AstraZeneca, bagging a payment of USD 30 million as an upfront licensing fee. AstraZeneca will now be taking care of the develo...
Read More...
Dec 14, 2017
Roche’s Phase 2 data; Celgene and Bluebird’s CAR-T therapy; Regeneron/Sanofi gets results; Allergan on women’s health
Positive Phase 2 data for Roche’s lymphoma ADC Roche’s antibody-drug conjugate for non-Hodgkin lymphoma polatuzumab has already received breakthrough designation from the USFDA and priority medicine status in the EU, and the expectations for the same are running pretty high. Phase 2 data presented by the company at...
Read More...
Nov 21, 2017
EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opioid lawsuit
EMA to relocate to Amsterdam by March 2019 Results of the Monday's vote for the European Medicines Agency relocation led to the emergence of Amsterdam as the new headquarters, beating the other finalists Milan and Copenhagen. Nineteen European Union member states submitted proposals for the EMA headquarters, and Mil...
Read More...
Nov 07, 2017
Teva sells generics; Valeant trades; Roche’s Genentech; J&J’s next potential frontier
Teva looks to sell generics in China through joint venture with Guangzhou Pharma As the world’s largest generics maker, Teva still doesn’t have much of a presence in China, even though it is a country relies heavily on generics. The Israeli company is looking to remedy that as Teva has said it “has no agreement with...
Read More...
Oct 16, 2017
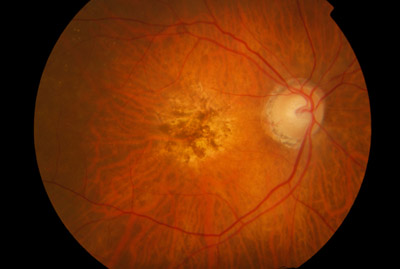
Wet AMD: A chronic eye condition with promising therapies
Wet AMD is a chronic eye disease that causes blurred vision or a blind spot in a person’s visual field. It is characterized by abnormal blood vessels that leak blood and fluids into the macula, a region of the eye that is responsible for sharp, central vision. Age-related macular degeneration (AMD) is a common eye c...
Read More...
Oct 10, 2017
Novo petitions FDA;J&J invests; Xarelto’s trial; Roche’s Tecentriq
Novo petitions FDA to require copies of Victoza to conduct clinical trials With one drugmaker already trying to pry patent protection off of Novo Nordisk’s blockbuster Victoza before its 2022 expiration, the insulin specialist has taken new steps to deter copies. The Danish drugmaker has filed a citizen petition arg...
Read More...






-Agonist.png)

