Traumatic Brain Injury
Nov 19, 2025
Neuroprotection Devices: Advancing Brain Protection Through Medical Innovation
The preservation of neuronal function following acute insults, such as ischemic events, traumatic brain injury (TBI), or cardiac arrest, represents one of the most significant challenges in modern critical care and neurology. Neuroprotection encompasses strategies and devices designed to minimize secondary injury a...
Read More...
Oct 06, 2025
Innovative RoA in Traumatic Brain Injury Treatment: Intranasal and Intrathecal Approaches
Traumatic brain injury should not be viewed solely as an acute condition; it can also manifest as a chronic disorder that adversely impacts quality of life and is associated with long-term health consequences. The development of services for TBI care can be guided by public health insights and policy awareness rega...
Read More...
Mar 07, 2024
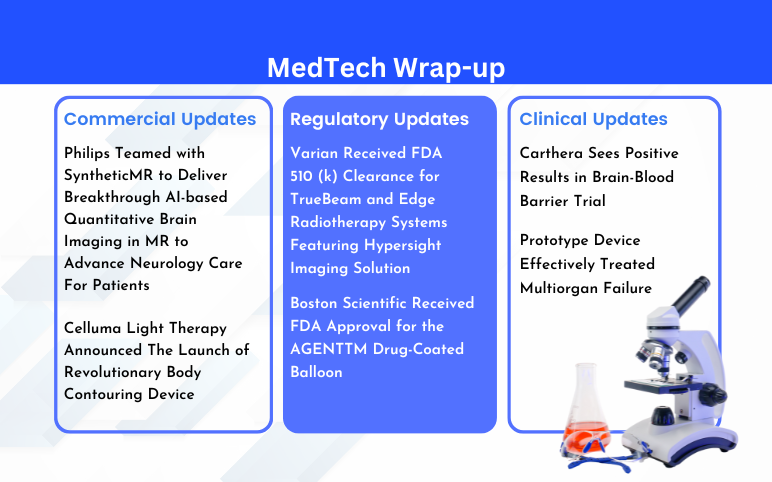
Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGENTTM Drug-Coated Balloon; Varian’s TrueBeam and Edge Radiotherapy Systems; Carthera’s Brain-Blood Barrier Trial; Prototype Device Effectively Treated Multiorgan Failure
Philips Teamed with SyntheticMR to Deliver Breakthrough AI-based Quantitative Brain Imaging in MR to Advance Neurology Care For Patients On March 01, 2024, Philips in collaboration with SyntheticMR announced the launch of Smart Quant Neuro 3D, a significant advancement in objective decision support for diag...
Read More...
Mar 09, 2023
Artica Systems’s Electric Whole-Body Cryotherapy Machines; Nevro’s Revolutionary HFX iQ™ Spinal Cord Stimulation System; COBRA Study of Stratus® Medical for Nimbus® Radiofrequency Ablation Device; Restore Medical’s CONTRABAND™ System; US FDA 510(k) Clearance for RS001 Cardio-respiratory Wearable; FDA Approval to Abbott’s Traumatic Brain Injury (TBI) Blood Test
New Commercial & Residential Electric Whole-Body Cryotherapy Machines Released by Artica Systems On March 2, 2023, Artica Systems, an American company with over 20 years of experience in the wellness industry announced the release of a new and innovative 100% electric, whole-body cryotherapy fleet mean...
Read More...
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...
Jan 18, 2022
Algernon’s NP-120 (ifenprodil); Pieris’s cinrebafusp alfa (PRS-343) clinical trial; Gaumard Scientific’s multidisciplinary patient simulator HAL S5301; Hekka Labs’s decentralized healthcare ecosystem
Algernon receives positive FDA feedback on Phase IIb chronic cough trial The US Food and Drug Administration (FDA) has given positive feedback for Algernon Pharmaceuticals’ Phase IIb clinical trial of NP-120 (ifenprodil) for chronic cough treatment. A receptor antagonist of N-methyl-D-aspartate (NMDA), ifenp...
Read More...
Jun 17, 2020
The Question That Remains Unanswered: What Might Be Causing Alzheimer’s?
50 million people globally have dementia, and out of all the dementia cases, Alzheimer’s Disease accounts for about 60–70% of cases, says the WHO. Alzheimer's Disease is a neurodegenerative disease, a common cause of dementia that affects behavior, memory, and thinking abilities of an individual. Mostly the symptom...
Read More...
Jan 30, 2020
Annovis announces IPO results; Conatus merges with Histogen; Novartis discontinues its generic journey
A Philadelphia-based pharmaceutical, Annovis Bio, has raised USD12 million by listing its 2 million shares at USD 6 each. The company has managed to net USD 2 million more by offering 40% more shares. The company plans to use the funds raised to address the unmet needs in the Alzheimer’s disease market...
Read More...







-Agonist.png)

