Innovative RoA in Traumatic Brain Injury Treatment: Intranasal and Intrathecal Approaches
Oct 06, 2025
Table of Contents
Traumatic brain injury should not be viewed solely as an acute condition; it can also manifest as a chronic disorder that adversely impacts quality of life and is associated with long-term health consequences. The development of services for TBI care can be guided by public health insights and policy awareness regarding the condition’s scope, diversity, and severity. Individuals with TBI often exhibit high rates of medication use, with research indicating that 45–85% of patients receive prescriptions for psychotropic and pain-relief medications.
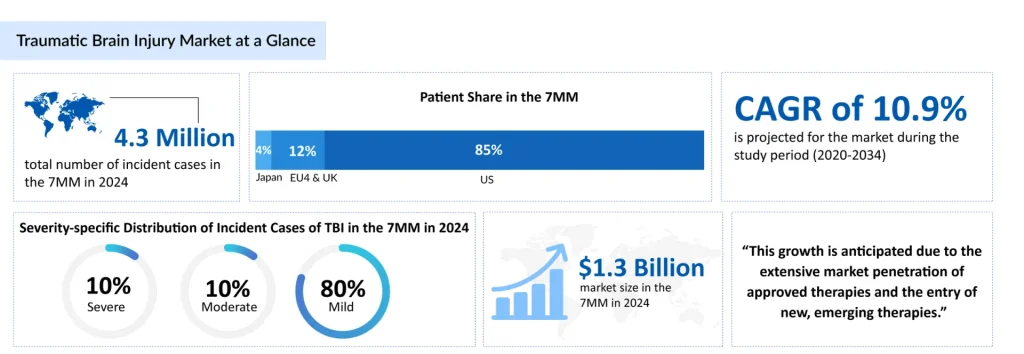
In 2024, approximately 4.3 million new TBI cases were reported across the leading markets, with projections suggesting this figure could rise to 4.5 million by 2034. Accurately assessing the full burden of TBI remains challenging due to underdiagnosis and underreporting; it is estimated that up to half of all TBIs, exceptionally mild cases, may go undetected or misidentified. Managing medications in TBI patients is complex yet highly rewarding, reflecting both the intricacy of the brain and the many uncertainties that remain in understanding its function.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath S...
- Artica Systems’s Electric Whole-Body Cryotherapy Machines; Nevro’s Revolutionary HFX iQ™ S...
- The Question That Remains Unanswered: What Might Be Causing Alzheimer’s?
- Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGE...
- Algernon’s NP-120 (ifenprodil); Pieris’s cinrebafusp alfa (PRS-343) clinical trial; Gaumard...
AKUUGO—The Only Approved TBI Treatment
At present, there are no FDA-approved therapies for TBI, and symptom management relies solely on off-label medications. These include psychostimulants, antidepressants, anticonvulsants, beta-blockers, and pain relievers, which target neuropsychiatric, neurocognitive, and motor impairments. Improvements in acute care have increased the number of TBI survivors, many of whom experience long-term disabilities, underscoring the urgent need for approved treatment options.
SanBio’s AKUUGO is an allogeneic cell therapy shown to be effective in treating chronic motor paralysis caused by TBI. It is the world’s first and only approved treatment for brain regeneration, and the company plans to expand its use to other central nervous system disorders with unmet medical needs.
AKUUGO received approval in July 2024 under Japan’s Sakigake Designation Program, obtaining conditional and time-limited marketing authorization for the human somatic stem cell product, “AKUUGO suspension for intracranial implantation,” specifically to improve chronic motor paralysis resulting from TBI.
In February 2025, SanBio successfully achieved the planned production yield for the second commercial batch of AKUUGO Suspension for Intracranial Implantation, thereby fulfilling the shipment requirements tied to its product approval in December 2024. AKUUGO has earned multiple regulatory recognitions, including Sakigake designation (April 2019) and priority review (January 2022) from Japan’s MHLW, orphan regenerative medicine designation (June 2020) from MHLW, RMAT designation (September 2019) from the US FDA, and ATMP designation (April 2019) from the EMA, reflecting its potential in treating chronic TBI. Shipment of AKUUGO is expected to begin in the second quarter of the fiscal year ending January 31, 2026 (May–July 2025), with no changes to the anticipated timeline.
Novel RoAs for TBI Treatment in Development
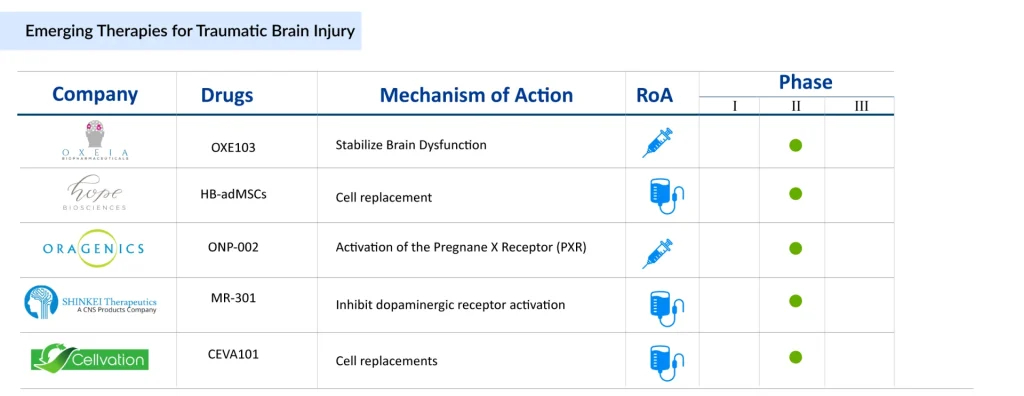
Companies are investigating different routes of administration for TBI treatment. Oragenics (ONP-002), Beyond Barriers Therapeutics (BBT-101), and Noveome (ST266) are exploring intranasal delivery, while Neuroplast (Neuro-Cells) is focusing on intrathecal injection as a potential approach. Apart from these, other TBI drugs in clinical trials include OXE103 (Oxeia Biopharmaceuticals), HB-AdMSCs (Hope Biosciences), CEVA101 (Cellvation), MR-301 (SHINKEI Therapeutics), and others.
Oxeia Biopharmaceuticals’ OXE103 is a synthetic form of human ghrelin, a naturally occurring hormone. It readily crosses the blood–brain barrier and supports the stabilization of metabolic and energy-related brain dysfunction following a concussion. Notably, OXE103 targets the hippocampus explicitly, a brain region essential for cognition and memory. In February 2025, the company announced plans to commence Phase II trials for OXE103 later that year.
Hope Biosciences’ HB-AdMSCs are mesenchymal stem cells derived from adipose tissue and cultured using Hope Biosciences’ proprietary platform. Hope Bio’s core expertise lies in stem cell culture and banking. In June 2024, the Department of Defense’s Office of CDMRP awarded UTHealth Houston a four-year, nearly USD 5 million clinical trial grant to investigate whether intravenous infusion of Hope Biosciences’ autologous HB-AdMSCs can reduce chronic neuro-inflammatory responses in severe TBI.
Oragenics’ ONP-002 is a first-in-class enantiomeric neurosteroid being developed to treat mild traumatic brain injury (mTBI or concussion). Its intellectual property covers the compound’s structure, synthetic methods, and applications. Preclinical studies in animal and cell culture models have shown that ONP-002 produces beneficial molecular and behavioral effects within hours of administration following neuronal injury.
Beyond Barriers Therapeutics is developing BBT-101, the first frontline therapy for mild to moderate concussions. Designed for rapid intervention immediately after injury, BBT-101 is administered intranasally to deliver the therapy directly to the affected brain region at the moment it is most needed.
The anticipated launch of these emerging TBI therapies are poised to transform the market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the traumatic brain injury treatment market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Recent Developments in the TBI Treatment Space
- In March 2025, Oragenics announced the submission of its IB application in preparation for its Phase II clinical trial using ONP-002 in Australia.
- In February 2025, SanBio announced that it had successfully secured the planned production yield for the second commercial production run of AKUUGO suspension for Intracranial Implantation, conducted to meet the shipment conditions required for obtaining product approval in December 2024.
- In February 2025, Oxeia Biopharmaceuticals announced plans to initiate Phase IIb trials for OXE103 later in the same year.
- In February 2025, Oragenics announced a strategic partnership with BRAINBox Solutions, a leader in multi-modality diagnostics for TBI.
- According to the company’s presentation in 2025, Oragenics is to initiate proof of concept Phase IIb for ONP-002 in the US with an open IND approved by the FDA. The company plans to file for accelerated approval in the fourth quarter of 2025 based on the Phase IIa findings. Additionally, the company anticipates submitting the Phase IIb findings by the third quarter of 2026.
What Lies Ahead in TBI Treatment?
The future of TBI treatment is poised for significant transformation, driven by advances in precision medicine, neurotechnology, and regenerative therapies. Researchers are increasingly focusing on individualized treatment approaches that account for the heterogeneity of TBI, recognizing that no two brain injuries are identical. According to DevleInsight analysis, the total market size of TBI in the 7MM was approximately USD 1.3 billion in 2024. This is estimated to increase by 2034 at a significant CAGR of 10.9%.
Emerging strategies include the use of biomarkers and advanced imaging to accurately assess injury severity and predict outcomes, enabling clinicians to tailor interventions more effectively. Novel pharmacological agents targeting neuroinflammation, oxidative stress, and excitotoxicity are being explored to limit secondary brain damage and improve recovery. Additionally, neurostimulation techniques, such as transcranial magnetic stimulation (TMS) and deep-brain stimulation (DBS), are showing promise in enhancing cognitive and motor recovery in TBI patients.
Regenerative medicine is another frontier with transformative potential. Stem cell therapies, including mesenchymal stem cells and induced pluripotent stem cell (iPSC)-derived neurons, are being investigated for their potential to repair damaged neural tissue and restore brain function. Advances in biomaterials and drug delivery systems are facilitating targeted therapeutic delivery across the blood-brain barrier, improving efficacy while minimizing systemic side effects.
Beyond biological interventions, digital health tools and AI-driven rehabilitation platforms are poised to revolutionize post-TBI care by offering personalized cognitive training, tracking recovery trajectories, and enhancing rehabilitation outcomes. Together, these innovations suggest a future where TBI treatment moves from primarily supportive care to precise, multifaceted strategies that improve functional recovery and long-term quality of life.

Downloads
Article in PDF
Recent Articles
- Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGE...
- Artica Systems’s Electric Whole-Body Cryotherapy Machines; Nevro’s Revolutionary HFX iQ™ S...
- CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath S...
- Neuroprotection Devices: Advancing Brain Protection Through Medical Innovation
- The Question That Remains Unanswered: What Might Be Causing Alzheimer’s?