USFDA
Apr 16, 2019
Notizia
FDA gives thumbs-up to Balversa The U.S. FDA has granted approval of Balversa (erdafitinib) to Belgium-based Janssen Pharmaceutica, for the treatment of advanced or metastatic bladder cancer. This drug is the first ever approved target-specific medication targeting susceptible FGFR genetic mutations, and for can...
Read More...
Nov 08, 2018
Gordon and Betty Moore Foundation gives $85M; Omeicos raises; Sanofi signs deal; Lilly grabs
Gordon and Betty Moore Foundation gives USD 85 Million to stop delayed, erroneous diagnostics The Gordon and Betty Moore Foundation is putting up money for improving diagnostics. The Foundation focuses on a plethora of areas, including those outside of healthcare, such as environmental conservation. It has an annua...
Read More...
Oct 12, 2018
Is Gene Therapy the Next Cancer Treatment Revolution?
Gene Therapy is now touted as a new revolution for cancer treatment. If the list of new cancer treatment methodologies is traversed, then the end seems to be farther and farther away. As a sequel to the relentless efforts by researchers across the world to formulate therapies better than the existing ones, new prosp...
Read More...
Mar 22, 2018
Business Cocktail
Merck and Eisai has entered into an Oncology Pact worth Up to USD 5.76 Billion Two major pharmaceutical companies (Merck & Eisai) has entered into a USD 5.76 Billion pact to develop and commercialize Lenvima (lenvatinib mesylate) both as a monotherapy and an in-combination therapy for treating multiple types of ...
Read More...
Jan 19, 2018
Familial Amyloid Polyneuropathy – An uncommon medical condition
Familial Amyloid Polyneuropathy (also known as transthyretin amyloidosis, TTR, and FAP) is a group of endangering multisystem disorders which are transmitted as an autosomal dominant trait. It is caused by the excessive accumulation of amyloid in the organs and tissues. The first identified cause of FAP is the Val30...
Read More...
Jan 10, 2018
Snippet
Gene therapy is back into play after a decade-long failed deliveries. 2017 was a golden year in gene therapy arena, various companies are aggressively developing gene therapy based treatments targeting life-threatening indications. Spark therapeutics got USFDA approval for the first-ever gene therapy based blindness...
Read More...
Oct 10, 2017
Novo petitions FDA;J&J invests; Xarelto’s trial; Roche’s Tecentriq
Novo petitions FDA to require copies of Victoza to conduct clinical trials With one drugmaker already trying to pry patent protection off of Novo Nordisk’s blockbuster Victoza before its 2022 expiration, the insulin specialist has taken new steps to deter copies. The Danish drugmaker has filed a citizen petition arg...
Read More...
Jul 25, 2017
Remicade’s launch; Endo cuts jobs; Analysts downgrade Pfizer; Corning $500M glass project
Samsung and Merck launch Remicade biosim at 35% discount After the first biosimilar of Johnson & Johnson’s big-selling immunology drug Remicade hit the market last year at a modest discount, another has come along with a much deeper pricing slash. Samsung and marketing partner Merck & Co. Monday launched the...
Read More...
May 30, 2017
Roche, Boehringer tout; Pfizer, Bristol-Myers got sued; FDA commissioner unveils; EpicGenetics expands
Roche, Boehringer tout new idiopathic pulmonary fibrosis analyses to boost their rival medications The idiopathic pulmonary fibrosis (IPF) market-share battle between Roche’s Esbriet and Boehringer Ingelheim’s Ofev hasn’t let up since the FDA green-lighted the medications in October 2014. And this week, both drugmak...
Read More...
May 24, 2017
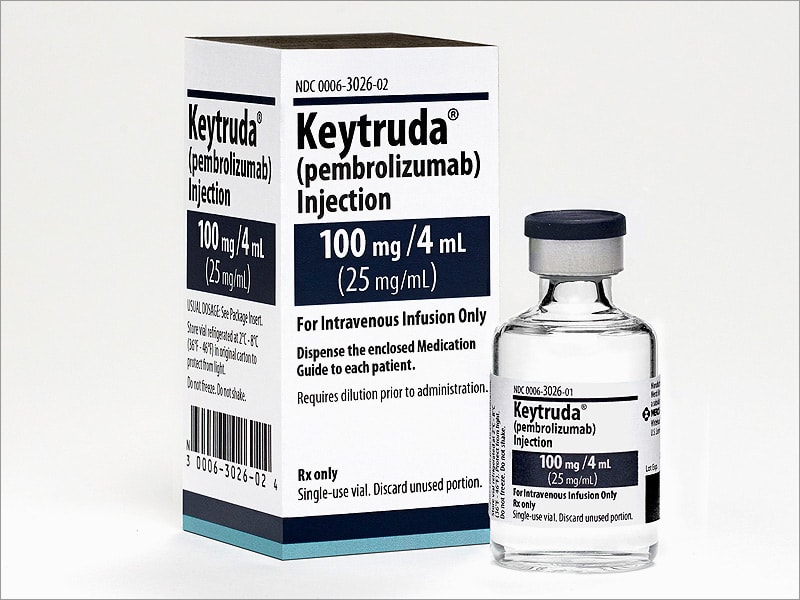
FDA grants accelerated approval to pembrolizumab
The U.S. Food and Drug Administration granted accelerated approval to pembrolizumab (KEYTRUDA, Merck & Co.) for adult and pediatric patients with unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment and...
Read More...




-Agonist.png)

