CLK Inhibitors Market Summary
-
The CLK Inhibitors market in the 7MM is projected to grow at a significant CAGR by 2034 in leading countries (US, EU4, UK and Japan).
CLK Inhibitors Market and Epidemiology Analysis
- The CLK Inhibitors market grow is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of CLK Inhibitors, and the increasing number of CLK inhibitors that are under clinical trials.
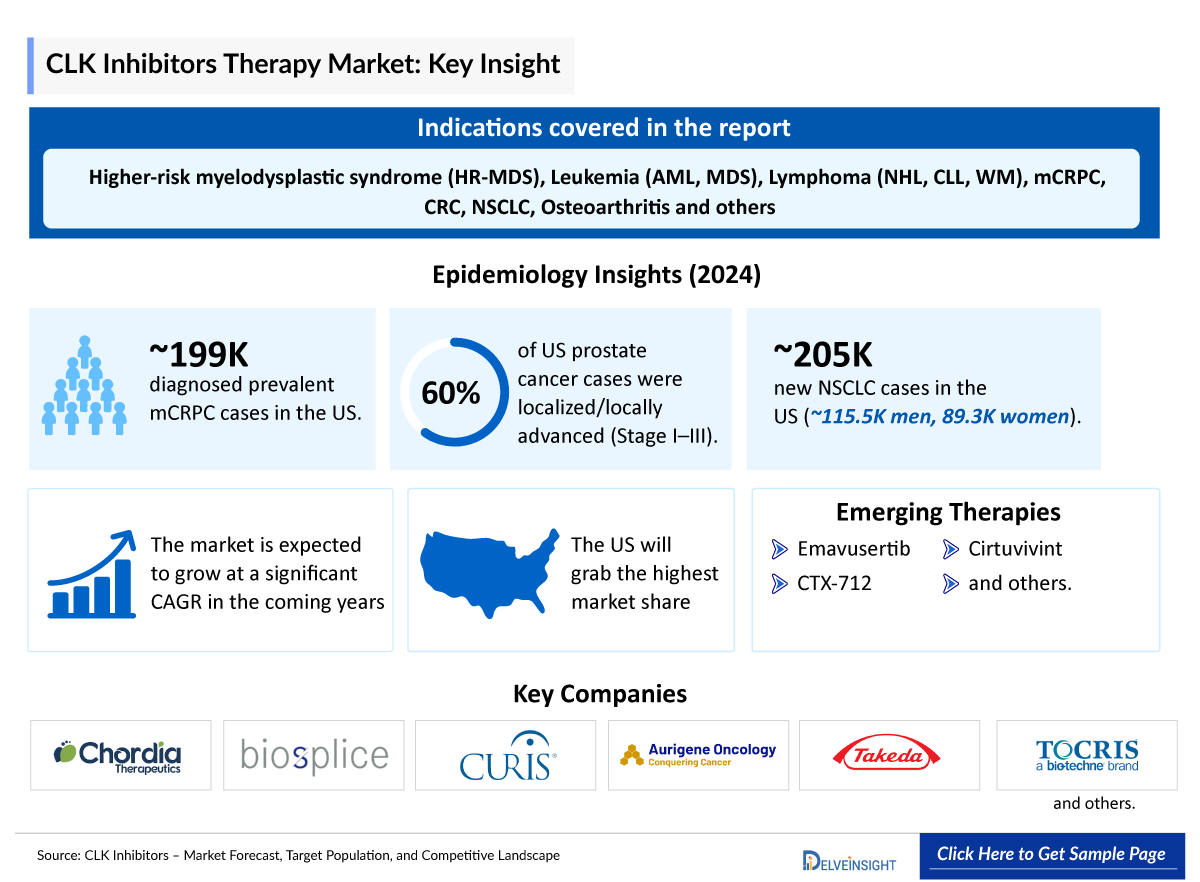
- In 2027, the United States CLK inhibitor market is expected to hold the largest share among the 7MM.
- CLK inhibitors offer a promising approach in addressing dysregulated alternative pre-mRNA splicing in cancer like non-small cell lung cancer, metastatic castration-resistant prostate cancer, and others, while also showing potential for treating musculoskeletal disorders such as osteoarthritis and degenerative disc disease.
- CLK inhibition might function as a novel pre‐mRNA splicing modulation‐based anti‐cancer strategy, especially for MYC‐driven cancers.
- Irregular expression of CLKs and the dysregulation of alternative splicing have been identified in several human diseases; therefore, CLKs have emerged as a new class of disease hallmarks.
- CLK1 and CLK2 are upregulated in various cancers including breast cancer, colorectal cancer, prostate cancer, and glioblastoma. CLK inhibitors have shown significant therapeutic effects in various human diseases including neurodegenerative diseases, inflammatory diseases, viral replication, and cancer.
- Currently there are no FDA-approved therapies that specifically target CLK.
- CLK Inhibitors Companies, including Biosplice Therapeutics and Chordia Therapeutics, among others are engaged in developing CLK inhibitors.
DelveInsight’s “CLK Inhibitors Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the CLK Inhibitor, historical and Competitive Landscape as well as the CLK Inhibitors market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The CLK Inhibitors market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM CLK Inhibitors market size from 2020 to 2034. The CLK Inhibitors market report also covers current CLK Inhibitor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
CLK Inhibitors Epidemiology |
Segmented by:
|
|
CLK Inhibitors Key Companies |
|
|
CLK Inhibitors Key Therapies |
|
|
CLK Inhibitors Market |
Segmented by:
|
|
CLK Inhibitors Market Analysis |
|
CLK Inhibitors Market: Understanding
CLK Inhibitors Overview
CLKs (Cdc2-like kinase) are dual specificity protein kinases that are involved in gene splicing regulation. The CLK family has four members, CLK1/STY, CLK2, CLK3, and CLK4. CLKs catalyze the phosphorylation of SR proteins, serine, and arginine-rich splicing factors 1-12 (SRSF1-12), which regulate the spliceosome molecular machinery. Compromised accuracy of alternative splicing can have a profound impact on human pathogenesis, in particular on tumor development and progression. Overexpression of active CLKs causes the redistribution of SR proteins within the nucleus and dissolution of speckles, dispersion of eIF4E nuclear speckles, complete redistribution of interchromatin granule clusters, and specific targeting of SRp55 for degradation by proteasome.
Based upon data provided by The Cancer Genome Atlas Program (TCGA), CLK1 is significantly overexpressed in many cancer types, including cholangiocarcinoma, colon adenocarcinoma, head and neck squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), prostate adenocarcinoma, rectum adenocarcinoma and stomach adenocarcinoma.
There are still limitations in the current understanding of CLKs. Firstly, the relationship between CLKs, immunotherapy, and the tumor microenvironment is currently unknown. Next, animal disease models, including CLKs conditional knockout or in situ disease models, are needed to fully understand the importance of CLKs in disease occurrence and development.
Further details related to country-based variations are provided in the report
CLK Inhibitor Epidemiology
The CLK Inhibitors epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases of selected indication for CLK Inhibitors, total eligible patient pool for CLK Inhibitors in selected indication, total treated cases in selected indication for CLK Inhibitors in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- In 2024, the United States accounted for the highest number of diagnosed prevalent cases of metastatic castration-resistant prostate cancer approximately 199,100.
- As per the estimates, in the US, the majority of the prostate cases were found to be localized/locally advanced cases (Stage I-III), comprising approximately 60% of total cases, while nearly 32% belonged to biochemical recurrence/ progressive cases.
- In the US, in 2024, there were approximately 204,800 new cases of NSCLC cancer, ~115,500 in men and ~89,300 in women.
- NSCLC is more common in males as compared to females. In the US, around 56% of males are diagnosed with NSCLC.
- Among the EU4 countries, the highest number of incident cases of colorectal cancer was observed in Germany in 2023, while Spain accounted for the lowest number of incident cases of colorectal cancer.
CLK Inhibitor Drug Chapters
The drug chapter segment of the CLK Inhibitor drugs market reports encloses a detailed analysis of CLK Inhibitor-marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the CLK Inhibitor’s clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Emerging CLK Inhibitors Drugs
Cirtuvivint (SM08502): Biosplice Therapeutics
Cirtuvivint (SM08502), developed by Biosplice Therapeutics, selectively targets dysregulated alternative pre-mRNA splicing in cancer cells. By inhibiting CLK and DYRK kinases, it induces tumor-specific changes in splicing events, reducing the expression of oncogenic proteins. This precision minimizes the impact on healthy tissues. Cirtuvivint shows promise in treating various cancers, including metastatic castration-resistant prostate cancer, non-small cell lung cancer, colorectal cancer, heme malignancies, and specific solid tumors. Currently, Cirtuvivint is in Phase I in its developmental process for metastatic castration-resistant prostate cancer, non-small cell lung cancer, and colorectal cancer. Additionally, it is advancing towards IND enabling selected heme malignancies and additional solid tumors, demonstrating its potential across diverse cancer types.
Lorecivivint (SM04690): Biosplice Therapeutics
Biosplice Therapeutics is advancing Lorecivivint as a potential therapy for knee, hip, and shoulder osteoarthritis, along with degenerative disc disease. This injectable small-molecule inhibitor of CLK and DYRK kinases has displayed encouraging outcomes in enhancing pain relief, functional capacity, and structural integrity. Biosplice Therapeutics, recently completed two Phase III trials, OA-07 long-term structure, pain and function study, and OA-21 short-term pain study. The final analysis of OA-07 results demonstrated statistically significant improvement in joint structure, WOMAC Pain, and WOMAC Function, highlighting the potential of lorecivivint to be a first-in-class, structure-modifying drug for the treatment of knee osteoarthritis. Preliminary analysis of OA-21 short-term pain results did not achieve statistical significance. The company has also initiated various IND-enabling studies of Lorecivivint for diseases such as osteoarthritis of the hip, osteoarthritis of the shoulder, and degenerative disc disease.
CTX-712: Chordia Therapeutics
CTX-712 is a first-in-class, orally available, highly potent, and selective small molecule inhibitor of CDC2-like kinase (CLK), a key regulator of RNA splicing. Preclinical data show that CTX-712 demonstrated anti-proliferative activity in in-vitro and in-vivo models derived from hematologic malignancies. A phase I study of CTX-712 was conducted to evaluate the safety and the preliminary efficacy of CTX-712 in patients with relapsed or refractory acute myeloid leukemia (AML) and higher-risk myelodysplastic syndrome (MDS).
|
Comparison of Key Emerging CLK Inhibitors | ||||
|
Product |
Company |
RoA |
Phase |
Indication |
|
Emavusertib (CA-4948) |
Curis/Aurigene Oncology |
IV |
II |
Acute Myeloid Leukemia (AML) and Myelodysplastic Syndromes (MDS) |
|
CTX-712 |
Chordia Therapeutics |
Oral |
I/II |
Acute Myeloid Leukemia (AML) and Myelodysplastic Syndromes (MDS) |
|
Cirtuvivint (SM08502) |
Biosplice Therapeutics |
IV |
I |
Solid tumors, Leukemia |
Note: Detailed emerging therapies assessment will be provided in the final report.
CLK Inhibitor Market Outlook
The CLK Inhibitors market is expected to grow in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of CLK Inhibitors, and the increasing number of CLK inhibitors that are under clinical trials.
CLK inhibitors offer a promising therapeutic avenue across a spectrum of diseases, ranging from various cancers like non-small cell lung cancer, colorectal cancer, ovarian cancer, metastatic castration-resistant prostate cancer, and acute myeloid leukemia to musculoskeletal conditions such as osteoarthritis of the hip, shoulder, and knee, as well as degenerative disc disease. Dysregulation of alternative pre-mRNA splicing, a common driver of tumor initiation, progression, and therapy resistance, underscores the significance of targeting CLK/DYRK kinases.
Cirtuvivint (SM08502), developed by Biosplice Therapeutics, emerges as a potent pan-inhibitor capable of modulating aberrant splicing events selectively in tumor cells while sparing healthy tissues. Its ability to reduce the expression of oncogenic proteins and inhibit tumorigenic regulatory systems holds promise for cancer treatment. Meanwhile, the orally available CLK inhibitor, CTX-712, demonstrates the potential to induce cancer cell death and anti-tumor effects in preclinical models. Additionally, Biosplice Therapeutics' exploration of Lorecivivint highlights the broader therapeutic potential of CLK inhibitors in musculoskeletal disorders. Through ongoing clinical trials and preclinical investigations, CLK inhibitors represent a novel and versatile approach to combating a diverse range of diseases, offering hope for improved patient outcomes and quality of life.
A few CLK Inhibitors companies, including Biosplice Therapeutics, Chordia Therapeutics, and others are involved in developing drugs for CLK Inhibitors for various indications such as prostate cancer, ovarian cancer, lung cancer, and others. Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of CLK Inhibitors and define their role in the therapy of cancer.
CLK Inhibitor Drugs Uptake
This section focuses on the uptake rate of potential emerging CLK Inhibitors expected to be launched in the CLK Inhibitors market during 2024–2034.
CLK Inhibitor Pipeline Development Activities
The CLK Inhibitors market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key CLK Inhibitors companies involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for CLK Inhibitors market growth over the forecasted period.
CLK Inhibitors Clinical Trials Activities
The CLK Inhibitors market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for CLK Inhibitor therapies.
Latest KOL Views on CLK Inhibitors
To keep up with current and future CLK Inhibitors market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on CLK Inhibitors' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Johns Hopkins Sidney Kimmel Cancer Center and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or CLK inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the CLK Inhibitors market and the unmet needs.
|
KOL Views |
|
“CLK inhibitors represent a promising new class of targeted therapies that modulate RNA splicing, a fundamental process in cancer cell proliferation and survival. Our preclinical and translational research with compounds such as T-025 and CC-671 demonstrates significant anti-tumor efficacy, particularly in cancers with high CLK2 expression or MYC amplification.” Phd, University of Chicago, US |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging CLK Inhibitors therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
CLK Inhibitors Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on CLK Inhibitor Clinical Trials
- In 2025, key milestones for the company include the planned publication of clinical data from its U.S. study and the initiation of Phase 2 trials in both the U.S. and Japan during the second half of the year.
- In May 2025, Biosplice announced the first patient dosed in a Phase 2 clinical trial of cirtuvivint for advanced soft-tissue sarcomas. This multicenter, open-label trial in Spain will evaluate cirtuvivint monotherapy in about 30 patients with disease progression after standard treatment.
- In April 2024, Biosplice presented positive Phase III results for Lorecivivint at the OARSI conference, indicating significant improvement in joint structure, WOMAC pain, and function in OA-07. However, preliminary analysis of OA-21’s short-term pain results did not achieve statistical significance. Biosplice presented its OA-07 results at the Osteoarthritis Research Society International (OARSI) World Congress, April 18-21, 2024, in Vienna, Austria. With the benefit of these clinical results, Biosplice intends to consult with the US Food and Drug Administration (FDA), as well as regulatory agencies in other countries, for further guidance on the path to marketing approval of lorecivivint.
Scope of the CLK Inhibitors Market Report
- The CLK Inhibitors market report covers a segment of key events, an executive summary, and a descriptive overview of the CLK Inhibitor, explaining its mechanism, and emerging CLK inhibitors.
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the CLK Inhibitor market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The CLK Inhibitors market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM CLK Inhibitor market.
CLK Inhibitor Market Report Insights
- CLK Inhibitor Targeted Patient Pool
- Therapeutic Approaches
- CLK Inhibitor Pipeline Analysis
- CLK Inhibitor Market Size
- CLK Inhibitors Market Trends
- Existing and future CLK Inhibitors Market Opportunity
CLK Inhibitor Market Report Key Strengths
- Eleven years Forecast
- The 7MM Coverage
- Key Cross Competition
- CLK Inhibitors Drugs Uptake
- Key CLK Inhibitors Market Forecast Assumptions
CLK Inhibitor Market Report Assessment
- Current Treatment Practices
- CLK Inhibitors Unmet Needs
- CLK Inhibitors Pipeline Product Profiles
- CLK Inhibitors Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the CLK Inhibitors Market Report
- What was the CLK Inhibitor market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for CLK Inhibitors market growth?
- Which CLK Inhibitors is going to be the largest contributor in 2034?
- Which is the most lucrative market for CLK Inhibitors?
- What are the pricing variations among different geographies for approved therapies?
- What are the risks, burdens, and unmet needs of treatment with CLK Inhibitors? What will be the growth opportunities across the 7MM for the patient population of CLK Inhibitors?
- What are the key factors hampering the growth of the CLK Inhibitor market?
- What are the indications for which CLK Inhibitors are being developed to overcome the limitations of existing treatments?
- What key designations have been granted to CLK Inhibitors?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to buy CLK Inhibitors Market Report
- The CLK Inhibitors market report will help develop business strategies by understanding the latest trends and changing dynamics driving the CLK Inhibitor Market.
- Understand the existing CLK Inhibitors market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved CLK Inhibitors in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming CLK Inhibitors companies in the CLK Inhibitors market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing CLK Inhibitors market so that the upcoming CLK Inhibitors companies can strengthen their development and launch strategy.