Spinal Muscular Dystrophy Treatment: A Deep Dive into Current Therapies and Future Prospects
May 12, 2025
Table of Contents
Spinal muscular atrophy (SMA) is the second most prevalent autosomal recessive disorder, with a carrier frequency of approximately 1 in 40 to 50 individuals and an estimated occurrence of 1 in 10,000 live births. The majority of SMA cases are caused by the deletion of a portion of the SMN1 gene, which impairs the production of the essential SMN protein.
As a rare genetic condition, spinal muscular atrophy incidence is best described in terms of birth prevalence—that is, the number of children born with the disorder over a specific time frame. The classical forms of SMA arise from mutations in both copies of the SMN1 gene, located on chromosome 5, leading to a significant deficiency of SMN protein.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
- Can Spinal Muscular Atrophy be treated by Protease Inhibitors?
- Top 12 Most Expensive Drugs in the US Healthcare Market
- Vedanta raises; Relay Therapeutics reels in; Gene therapy for SMA
- Watershed Moment for Cell Therapies and Complicated Journey of Gene Therapies in Japan
Another gene, SMN2, also contributes to SMN protein production; however, the protein it generates is usually incomplete, and only about 10% to 20% of it is functional. It is estimated that around 60% of infants diagnosed with SMA have type I, the most severe form, while types II and III account for the remaining 40%.
There is currently no definitive cure for spinal muscular atrophy. Spinal muscular atrophy treatment focuses on symptom management and complication prevention.
FDA-Approved Therapies for Spinal Muscular Atrophy Treatment
The approval of three treatments for spinal muscular atrophy, namely, SPINRAZA (Ionis/Biogen), EVRYSDI (Roche), and the groundbreaking gene therapy ZOLGENSMA (Novartis), has significantly addressed several unmet needs, offering patients more personalized therapeutic options. Still, managing SMA effectively goes beyond these medications. It requires a robust, multidisciplinary approach that encompasses supportive care to reduce complications and improve quality of life for both patients and their families. This integrative care model includes input from neurology, physical therapy, nutrition, and other specialties to support long-term outcomes.
While symptomatic treatments are available, a comprehensive management plan also emphasizes key areas such as nutritional support, respiratory evaluation, respiratory muscle care, orthopedic interventions, rehabilitation, and select off-label medications—all contributing to a well-rounded care strategy for SMA patients.
ZOLGENSMA, developed by AveXis, a Novartis company, has received FDA approval as a one-time gene therapy for children under 2 years of age with SMA. It works by targeting the root genetic cause—replacing the function of the missing or faulty SMN1 gene to halt disease progression. Delivered through a single infusion, ZOLGENSMA introduces a functional SMN gene to motor neuron cells, helping maintain muscle function.
The therapy uses a vector derived from AAV9, which is not known to cause illness. Before administering ZOLGENSMA, physicians must check that the child’s AAV9 antibody levels are within an acceptable range via a blood test to ensure effective gene therapy delivery.
SPINRAZA enhances SMN protein production by using an antisense oligonucleotide to modify the splicing of the SMN2 gene. EVRYSDI, a small molecule, also promotes functional SMN protein production by influencing SMN2 splicing.
Of the three FDA-approved spinal muscular atrophy treatments, SPINRAZA and ZOLGENSMA have emerged as blockbuster therapies, with SPINRAZA securing a major portion of the market across the 7MM. These innovative spinal muscular atrophy drugs have revolutionized the spinal muscular atrophy treatment and generated substantial revenues for the companies behind them.
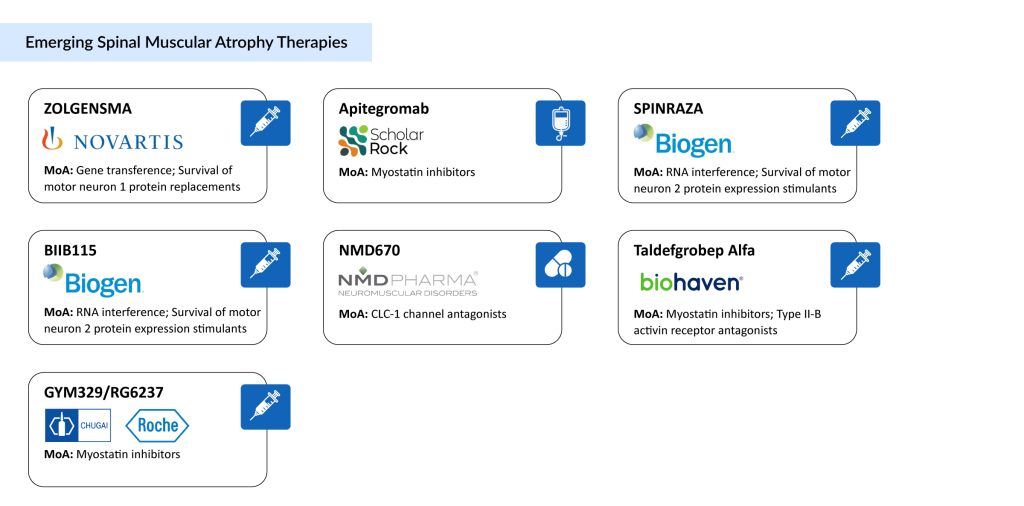
Spinal Muscular Atrophy Pipeline Therapies: The Race for a Cure
Since 2016, the FDA has approved three treatments that modify the course of spinal muscular atrophy, marking a major shift in managing the disease. Over the past decade, clinical approaches to treating SMA have been transformed, with several companies such as Novartis, Scholar Rock, and Biogen advancing innovative therapies through clinical trials. Notable spinal muscular atrophy pipeline candidates include Novartis’ intrathecal ZOLGENSMA (OAV101 IT), Scholar Rock’s Apitegromab, NMD Pharma’s NMD670, Biogen’s higher-dose SPINRAZA and BIIB115, Biohaven’s Taldefgrobep Alfa, and Chugai/Roche’s GYM329/RG6237, among others.
The emergence of new spinal muscular atrophy drugs is poised to significantly transform the rare disease treatment landscape and have a profound impact on the market. Their launch will likely expand the addressable patient pool due to enhanced newborn screening and increased disease awareness. Additionally, the competitive market environment may drive pricing dynamics, payer negotiations, and access strategies, while also stimulating further innovation in neuromuscular and genetic disorders. Overall, these therapies are expected to reshape standards of care and redefine long-term expectations for SMA management.
Recent Breakthroughs in Spinal Muscular Atrophy Treatment
- In March 2025, Scholar Rock announced that the FDA had accepted its Biologics License Application (BLA) for apitegromab. This investigational muscle-targeted treatment aims to improve motor function in people with spinal muscular atrophy (SMA) who are already receiving SMN-targeted treatment.
- In February 2025, Genentech, a member of the Roche Group, announced that the FDA approved a New Drug Application (NDA) for an EVRYSDI (risdiplam) tablet for individuals living with spinal muscular atrophy.
- In January 2025, Scholar Rock announced the submission of a Biologics License Application (BLA) to the FDA for apitegromab. This muscle-targeted therapy aims to provide significant motor function improvements in patients with SMA who are undergoing SMN-targeted treatments.
- In January 2025, Biogen announced that the FDA had accepted its supplemental New Drug Application (sNDA) and the EMA had validated the application for a higher dose regimen of nusinersen for spinal muscular atrophy.
- In September 2024, Biogen Inc. announced positive topline data from Part B of the Phase II/III DEVOTE study. This pivotal trial evaluated a higher dose regimen of nusinersen in treatment-naïve, symptomatic infants with spinal muscular atrophy. The new regimen includes a rapid loading phase with two 50 mg doses 14 days apart, followed by a higher maintenance dose of 28 mg every 4 months, compared to the current nusinersen regimen (SPINRAZA).
What’s in Store for Spinal Muscular Atrophy Treatment Advancements?
The future of spinal muscular atrophy treatment is increasingly promising, driven by advancements in gene therapy, molecular therapies, and a deeper understanding of the disease at a genetic and cellular level. SMA, caused by mutations in the SMN1 gene, leads to progressive muscle weakness and atrophy. Historically, treatment options were limited, but recent breakthroughs have dramatically changed the landscape, offering new hope for affected individuals.
The success of gene therapies in neuromuscular disorders, such as ZOLGENSMA, which replaces the defective SMN1 gene, represents a major milestone in SMA care. With such treatments, we’re seeing significant improvements in survival rates and motor function, even for infants diagnosed with the most severe forms of the disease.
Moreover, advancements in antisense oligonucleotide (ASO) therapies, such as SPINRAZA and EVRYSDI, have shown tremendous promise in improving the quality of life for SMA patients. These therapies work by modifying the splicing of the SMN2 gene to produce more functional SMN protein, which is crucial for motor neuron survival.
While these therapies have brought hope to patients across different age groups, researchers continue to explore ways to enhance their effectiveness, reduce side effects, and increase accessibility. The ongoing studies focus on optimizing the treatment regimen, understanding the long-term outcomes, and potentially developing combination therapies that could further improve results.
As the field progresses, we’re likely to see more personalized approaches to SMA treatment, including gene editing tools like CRISPR-Cas9 that could offer highly targeted, one-time interventions for genetic correction. Furthermore, advancements in biomarker discovery could allow for earlier detection of SMA and better monitoring of treatment efficacy, ensuring that therapies are tailored to individual patient needs.
As these innovative treatments become more accessible, we can expect a shift towards improved outcomes in both the early and late stages of the disease. While challenges remain, particularly in terms of affordability and long-term sustainability, the future of SMA treatment holds immense potential to transform patient lives and change the trajectory of the disease.

Downloads
Article in PDF