Nucleic Acids and Gene Therapies in Neuromuscular Disorders: Next-Generation Therapeutic Strategies
Aug 23, 2024
Neuromuscular disorders (NMD) encompass a broad spectrum of conditions impacting the peripheral nervous system, which comprises all motor and sensory nerves linking the brain and spinal cord to the body. These disorders comprise various classes of diseases, such as muscular dystrophies, myopathies, motor neuron diseases, ion channel diseases, mitochondrial diseases, neuromuscular diseases, and peripheral nerve diseases. Several conditions fall under the classification of neuromuscular disorders, with prominent examples including Duchenne muscular dystrophy (DMD), spinal muscular atrophy (SMA), multiple sclerosis, and others.
The prevalence of neuromuscular disorders has gained increasing recognition in the medical landscape, emphasizing the impact of these conditions on the intricate interplay between the nervous system and muscles, with a growing understanding of their diverse manifestations and the need for comprehensive management strategies.
DelveInsight’s analysis reveals that the overall diagnosed prevalent multiple sclerosis population in the 7MM was reported as 1.2 million in 2023. Within this, the diagnosed prevalent population of multiple sclerosis patients in the United States specifically was identified to be 723K in the same year. Another analysis by DelveInsight reveals that the overall prevalent population of DMD in the 7MM reached 31K in 2023. As per the estimates, in the United States, in 2023, the highest proportion of age-specific cases were observed in 5-9 years, followed by age groups of 10-14 years and 15-20 years.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opio...
- What are your views on Gene Therapy?
- Watershed Moment for Cell Therapies and Complicated Journey of Gene Therapies in Japan
- Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to S...
- Are you ready to embrace Gene Therapy?
Presently, there is no definitive solution for addressing neuromuscular disorders. Ongoing research is focused on genetic therapies, novel medications, and nucleic acids such as DNA and RNA, aiming to discover a cure. Meanwhile, the current approach involves managing symptoms, slowing the advancement of the disease, and improving patients’ quality of life through the use of medications, physical therapy, occupational therapy, and surgery when deemed necessary.

Approved Therapies Available for Neuromuscular Disorders Treatment
ZOLGENSMA (Onasemnogene abeparvovec-xioi) is the first and only gene therapy that is currently approved by the FDA for the treatment of SMA. Along with gene therapy, the FDA had previously approved SPINRAZA (nusinersen) which is an RNA-based antisense oligonucleotide being used for the treatment of SMA. SPINRAZA is an antisense oligonucleotide designed for the management of spinal muscular atrophy in both pediatric and adult populations. Ionis Pharmaceuticals developed SPINRAZA, and it received priority review approval from the FDA in December 2016. Additionally, the European Medicines Agency (EMA) granted acceptance of the marketing authorization application (MAA) for SPINRAZA in October 2016.
Other than these two drugs, the FDA has also approved an mRNA splicing modifier that is EVRYSDI (Risdiplam) which was the first drug developed for the treatment of SMA. EVRYSDI represents a groundbreaking achievement as the first and only oral medication designed for addressing spinal muscular atrophy in individuals aged two months and beyond. Genentech, in collaboration with the SMA Foundation and PTC Therapeutics, spearheaded the development of this medication. The FDA approved EVRYSDI for the treatment of SMA in both adults and children aged two months and older in August 2020. Notably, EVRYSDI received orphan drug designation in January 2017, and the FDA bestowed fast-track designation in April 2017.
In recent news in June 2024, it was highlighted that ever since the approval, EVRYSDI has shown remarkable long-term efficacy in treating spinal muscular atrophy (SMA). After five years of treatment, 91% of patients remained alive, 81% lived without needing permanent ventilation, and 59% could sit independently for at least 30 seconds. The data, presented at the Cure SMA Research and Clinical Care Meeting, also highlighted that seven children could stand—three with support and four unaided—while six were able to walk with assistance by the end of the five-year period. Roche, in partnership with the SMA Foundation and PTC Therapeutics, spearheads the clinical development of EVRYSDI. The drug has been approved in over 100 countries, with additional submissions currently under review in 13 more nations.
AMONDYS 45 (casimersen) injection is an antisense oligonucleotide prescribed for individuals diagnosed with Duchenne muscular dystrophy. It represents the inaugural treatment approved by the FDA for DMD patients possessing a mutation that allows for the skipping of exon 45. The FDA granted accelerated approval to AMONDYS 45 in February 2021 for the treatment of DMD.
EXONDYS 51 (eteplirsen), another approved therapy for neuromuscular disorder treatment developed by Sarepta Therapeutics, is an exon-skipping treatment designed for individuals with Duchenne muscular dystrophy possessing genetic mutations suitable for exon 51 skipping. It aims to tackle the root cause of Duchenne by enhancing the production of dystrophin protein in muscle cells. The accelerated approval pathway of the FDA approved EXONDYS 51 in September 2016.
VILTEPSO (viltolarsen), an antisense oligonucleotide developed by NS Pharma, is utilized for the treatment of Duchenne muscular dystrophy in individuals with a confirmed mutation of the DMD gene amenable to exon 53 skipping. This groundbreaking therapy marks the first and sole exon 53 skipping treatment to demonstrate increased dystrophin levels in children as young as four years old. The FDA received an NDA for VILTEPSO in October 2019 and accorded the drug priority review status in February 2020. Subsequently, accelerated approval was granted to the drug in August 2020.Moreover, on June 22, 2023, the FDA approved Sarepta’s ELEVIDYS, marking the first gene therapy authorized for Duchenne muscular dystrophy treatment. Priced at $3.2 million as a single-dose treatment, the approval followed a delay in late May. During that period, FDA officials and consultants had raised concerns about the efficacy of Sarepta’s research. Specifically, SRP-9001, the gene therapy, seemed to have a limited impact on muscular performance, affecting only certain individuals.
With the approval of ELEVIDYS, the neuromuscular disorder treatment landscape is undergoing some major changes and will likely witness a boost in the coming years. Nevertheless, a significant demand exists for novel and efficacious treatments to address the unmet needs in treating neurological disorders. However, there have been recent efforts in the field of managing neurological disorders, as evidenced by the interest displayed by influential stakeholders. Consequently, as promising therapies are currently under exploration, the market landscape for this ailment is expected to undergo positive changes. This should stimulate insurance reimbursement, garner support from medical professionals, enhance patient compliance, and ultimately give rise to a vast and untapped sector.
Promising Nucleic Acids and Gene Therapies for Neuromuscular Disorders Treatment in Pipeline
The nucleic acid and gene therapies in neuromuscular disorders treatment market landscape is expected to transform, driven by global efforts from companies dedicated to advancing new gene therapy solutions for diverse medical conditions. Major industry players with their lead assets such as Pfizer (PF-06939926 (fordadistrogene movaparvovec)), Novartis (Branaplam (LMI070 and NVS-SM1)), Ionis Pharmaceuticals (ION363), Helixmith (Engensis (VM202)), and others are actively engaged in the development of gene therapies targeting conditions such as DMD, SMA, MS, ALS, and others.
PF-06939926, also known as fordadistrogene movaparvovec, is a Pfizer-developed investigational therapeutic. It involves a recombinant adeno-associated virus serotype 9 (rAAV9) capsid that carries a truncated version of the human dystrophin gene (mini-dystrophin). This genetic material is regulated by a human muscle-specific promoter. The choice of the rAAV9 capsid as the delivery vector is based on its potential to specifically target muscle tissue. Currently, the drug is in the recruiting phase of its Phase III development, aimed at treating patients with Duchenne muscular dystrophy (DMD).
In June 2024, Pfizer announced that its Phase III CIFFREO study of fordadistrogene movaparvovec (PF-06939926) failed to meet its primary endpoint. The company reported that the gene therapy’s safety profile was deemed “manageable,” with adverse events primarily mild to moderate, and serious adverse events responding well to clinical management. This follows a recent fatality in the Phase II DAYLIGHT study, which led to a dosing pause in CIFFREO. Meanwhile, Pfizer’s other gene therapy, fidanacogene elaparvovec-dzkt (Beqvez), was approved by the FDA for hemophilia B treatment on April 26, 2024, and by Health Canada in January 2024. Beqvez is indicated for adults with moderate to severe hemophilia B who have specific clinical needs and do not possess neutralizing antibodies to the AAVRh74var capsid.
Branaplam, also known as LMI070 and NVS-SM1, is a pyridazine derivative undergoing investigation as an experimental medication. Originally formulated by Novartis to address spinal muscular atrophy (SMA), this compound works by enhancing the production of functional survival of motor neuron protein derived from the SMN2 gene, achieved through modifications to its splicing pattern. Clinical trials exploring its efficacy in treating SMA, particularly in children with SMA type 1, were conducted from 2014 until the program’s discontinuation in 2021. Presently, the drug is in an active Phase I/II status but is not actively recruiting participants for the treatment of SMA patients.
ION363 is a prospective antisense medication developed by Ionis Pharmaceuticals to diminish the synthesis of the Fused in Sarcoma (FUS) protein. Referred to as Jacifusen, albeit not yet bestowed with an official United States Adopted Name (USAN), this drug pays tribute to Jaci Hermstad, the inaugural patient treated through an expanded access initiative. The focus of ION363’s development is on individuals afflicted with an uncommon hereditary manifestation of amyotrophic lateral sclerosis (ALS) stemming from mutations in the FUS gene. The mutated FUS triggers motor neuron degeneration via a deleterious gain-of-function mechanism, leading to the aggregation of the mutant FUS protein in the motor neurons of patients. The application of antisense technology to curtail the mutant FUS protein in a FUS-ALS mouse model has demonstrated its efficacy in preventing the loss of motor neurons. The working hypothesis is that diminishing the FUS protein holds the potential to either reverse or impede the progression of the disease in individuals with FUS-ALS.
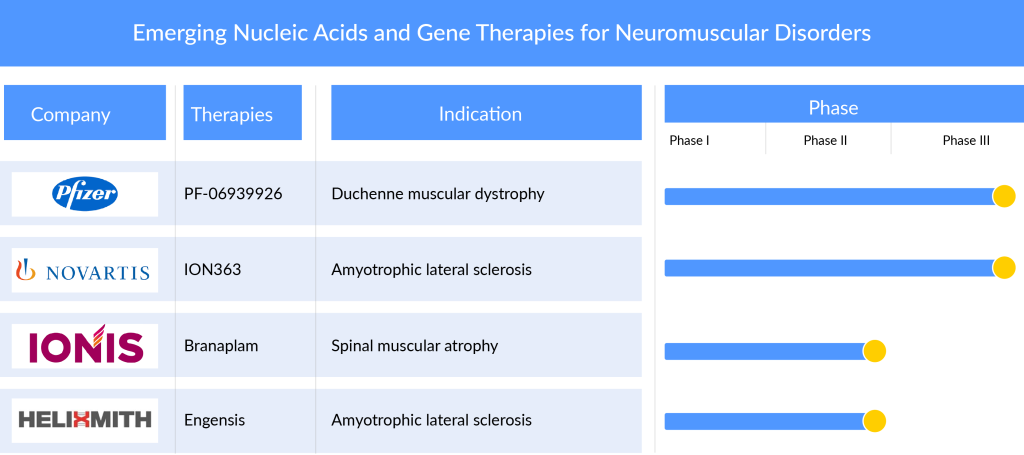
Engensis (VM202) represents an innovative gene therapy medication currently under development by Helixmith. This groundbreaking treatment focuses on fundamental healing through tissue regeneration. Utilizing a non-viral gene therapy approach, Engensis employs a plasmid—a small, circular DNA molecule—to transport the hepatocyte growth factor (HGF) gene directly to nerve and nerve-supporting cells in patients.
Throughout ten clinical trials spanning six different diseases and conditions, more than 500 patients have undergone treatment with Engensis. Previous clinical studies indicate that the therapy is well-tolerated, showcasing the potential to provide lasting relief from pain and symptoms in various medical scenarios. Beyond addressing pain, Engensis is specifically designed to target the root causes of neuropathy through its anticipated angiogenic and neurodegenerative properties.
The United States Food and Drug Administration (FDA) acknowledged Engensis’s potential to address an unmet medical need in 2018 by granting it the designation of Regenerative Medicine Advanced Therapy (RMAT). This marked a significant milestone, as Engensis became the first RMAT-designated gene therapy aimed at a prevalent disease affecting over one million patients.
The Road Ahead: Toward a New Era in Neuromuscular Disorder Treatment
As we stand at the threshold of a new era in medical science, the road ahead holds promising prospects for the treatment of neuromuscular disorders. Advances in neurology, genetics, and therapeutic interventions have set the stage for innovative approaches that aim to alleviate the burdens imposed by these complex conditions. With a deeper understanding of the genetic underpinnings of neuromuscular disorders, researchers are now exploring targeted gene therapies and gene-editing techniques to address the root causes at a molecular level.
Furthermore, the advent of precision medicine, fueled by advancements in personalized genomic medicine, offers a tailored approach to treatment. By identifying specific genetic mutations and variations in individuals, healthcare professionals can design personalized therapeutic strategies that target the unique aspects of each patient’s condition. This individualized approach holds the potential to revolutionize the effectiveness of treatments while minimizing adverse effects.
As we navigate the road ahead, collaboration between multidisciplinary teams, including clinicians, geneticists, neuroscientists, and technology developers, will be crucial. Embracing cutting-edge technologies such as CRISPR-Cas9 gene editing, RNA-based therapies, and neuromodulation devices will likely play a pivotal role in shaping the landscape of neuromuscular disorder treatment. The collective efforts of researchers, healthcare providers, and the pharmaceutical industry are paving the way for a new era where innovative therapies offer hope for improved outcomes and an enhanced quality of life for individuals grappling with neuromuscular disorders.

Downloads
Article in PDF
Recent Articles
- South Korean researchers influencing lift of human-embryo restrictions
- Bristol-Myers Squibb’s Opdivo & Yervoy Combo Trial; Sarepta’s Gene Therapy SRP-9001 for DMD;...
- Gilead Buys Out Rights to Cancer Therapy from Jounce; FDA Places Clinical Hold on Biogen’s Orelab...
- Gene Therapy to “Assassinate” HIV virus
- Hemophilia B Market: How Pipeline Therapies are Transforming the Treatment Hemisphere?



