Evolving Landscape of Anti-GPC3 Targeted Therapies: ADCs, CAR-T Cells, and Beyond
Dec 05, 2025
Table of Contents
Glypican-3 (GPC3) has emerged as one of the most promising therapeutic targets in oncology, particularly in hepatocellular carcinoma and select solid tumors. As a cell surface heparan sulfate proteoglycan that is overexpressed in approximately 75% of hepatocellular carcinoma cases while remaining virtually absent in normal adult tissues, GPC3 represents an ideal target for precision medicine approaches. The evolution of anti-GPC3-targeted therapies, from early monoclonal antibodies to sophisticated next-generation formats, marks a transformative shift in cancer treatment strategy.
Understanding Glypican-3: The Target Molecule
Glypican-3 is a cell surface heparan sulfate proteoglycan that plays a crucial role in cell growth, differentiation, and signaling pathways, including the Wnt, Hedgehog, and FGF pathways. It is highly expressed in several malignancies, most notably HCC, while showing limited expression in normal adult tissues, making it an attractive therapeutic target.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Driving factors boosting the Hepatocellular Carcinoma Market
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- How is Nuclear Medicine Bringing Groundbreaking Transformation in the Healthcare Industry?
- Japan Manages to Stand Apart from Global Hepato Cellular Carcinoma Crowd
- Cellenkos’ CK0801 Granted FDA Orphan Drug Status for Aplastic Anemia; Mirum’s LIVMARLI Gets FDA N...
Biological Significance and Expression Pattern
GPC3 plays a crucial role in cell growth regulation through the Wnt signaling pathway. During normal development, GPC3 is widely expressed across multiple tissues, but this expression is silenced in adult tissues through DNA methylation, resulting in minimal or undetectable levels in healthy adult organs. This pattern makes GPC3 uniquely suited for targeted therapy development—it appears almost exclusively in cancer cells while being largely absent from normal tissues, thereby minimizing off-tumor effects.
Diagnostic and Prognostic Value
GPC3 has transitioned from being solely a therapeutic target to becoming an essential biomarker for cancer diagnosis and prognosis. Immunohistochemical detection of GPC3 is more sensitive and specific for HCC diagnosis than traditional markers, such as alpha-fetoprotein (AFP). Serum GPC3 levels, particularly when combined with AFP, demonstrate enhanced sensitivity for early detection of HCC. Furthermore, GPC3 expression has been identified as an independent prognostic factor associated with poor disease-free survival in early-stage HCC, with GPC3-positive patients exhibiting significantly lower 5-year disease-free survival rates (approximately 27%) compared to GPC3-negative patients (approximately 62%).
Anti-GPC3 Targeted Therapies Target Patient Populations
Across the seven major markets (the United States, Germany, France, Italy, Spain, the United Kingdom, and Japan), the target patient populations present significant market opportunities. The highest GPC3 expression rates are observed in hepatocellular carcinoma. Squamous cell lung cancer adds approximately 55,000 US cases in 2024, while Merkel cell carcinoma accounts for around 4,000 cases.
Additional potential indications include gastric cancer, yolk sac tumors, liposarcoma, and other GPC3-expressing malignancies. Notably, global variability in GPC3 biomarker testing rates highlights a substantial opportunity for developing diagnostic platforms to support patient stratification and optimized therapy selection.
The Evolution of Anti-GPC3 Therapeutic Approaches
The journey of anti-GPC3 therapeutics began with monoclonal antibodies designed to leverage antibody-dependent cellular cytotoxicity (ADCC). Codrituzumab (GC33), developed by Chugai Pharmaceutical, was the first GPC3-targeting agent to enter clinical trials. Despite localizing effectively to HCC tumors in vivo and demonstrating acceptable safety profiles, codrituzumab failed to translate its strong preclinical promise into a clinically meaningful benefit. This critical setback provided essential insights into the fundamental challenges of solid tumor targeting, highlighting two crucial limitations: antibodies lack sufficient potency in solid tumors, and success requires biomarker-driven patient selection strategies with optimized dosing approaches.
Other notable first-generation programs that did not advance included SAR444200, AZD5851, and ERY974, each of which contributed valuable data that informed subsequent therapeutic development. The high attrition rate of early GPC3-targeted programs reflects the inherent complexity of translating strong preclinical promise into clinical efficacy.
Next-Generation Anti-GPC3 Therapeutic Modalities
Currently, there are no approved therapies targeting GPC3, despite its strong rationale as a biomarker and therapeutic target in HCC and select solid tumors. Multiple approaches, including monoclonal antibodies, T-cell engagers, CAR-T therapies, and radiopharmaceuticals, have been explored.
Antibody-Drug Conjugates (ADCs)
ADCs represent a sophisticated approach that combines the tumor-targeting specificity of monoclonal antibodies with the cytotoxic potency of chemotherapy payloads. These engineered molecules deliver potent cytotoxic agents directly to GPC3-positive cancer cells, resulting in the selective killing of tumor cells while sparing normal tissues.
Preclinical studies have demonstrated the remarkable efficacy of GPC3-targeted ADCs. When tested against HCC cell lines, these conjugates exhibited cytotoxicity in the picomolar range against GPC3-positive cells, while remaining largely inactive against GPC3-negative cells. In vivo models demonstrated significant tumor regression following single-dose administration, even in tumors exhibiting low or heterogeneous GPC3 expression—a critical advantage for addressing the tumor heterogeneity challenge inherent in solid malignancies.
ZW251, developed by Zymeworks, exemplifies the ADC approach in the current pipeline. This bispecific antibody-drug conjugate has demonstrated strong antitumor activity in hepatocellular carcinoma patient-derived xenograft (PDX) models. In July 2025, Zymeworks announced FDA clearance of the Investigational New Drug application for ZW251, marking a significant milestone toward clinical evaluation.
T-Cell Engagers: Redirecting Immune Cells to Cancer
T-cell engagers represent a paradigm shift in anti-GPC3 therapy by leveraging the patient’s own immune system to recognize and eliminate tumor cells. Unlike conventional monoclonal antibodies that rely on the patient’s existing immune response, T-cell engagers actively recruit and redirect cytotoxic T cells to GPC3-positive tumor cells through bispecific or trispecific antibody designs.
Bispecific Antibodies
These molecules simultaneously bind to GPC3 on tumor cells and CD3 on T cells, forming a bridge that brings T cells into direct contact with cancer cells. The physical crosslinking of T cells and tumor cells overcomes limitations of conventional antigen recognition, including major histocompatibility complex (MHC) downregulation and low-affinity T-cell receptor binding.
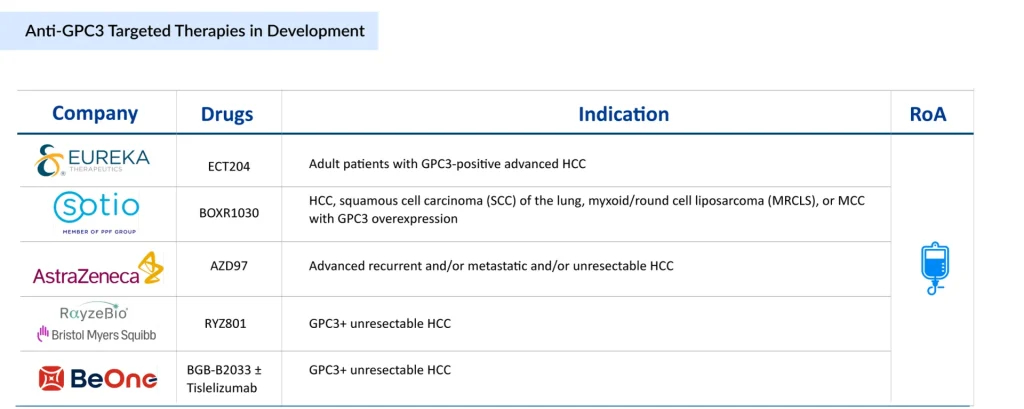
BGB-B2033, developed by BeOne Medicines (BeiGene), represents an innovative bispecific approach. This molecule triggers 4-1BB activation on CD8+ T cells exclusively in the presence of GPC3-positive tumor cells, thereby promoting targeted immune-mediated destruction of tumor cells. The 4-1BB costimulatory pathway activation enhances T-cell proliferation and survival, leading to more potent and sustained anti-tumor activity. BGB-B2033 is under clinical investigation in Phase I for multiple GPC3-expressing malignancies, including advanced HCC, AFP-producing gastric cancer, extragonadal yolk sac tumors, and GPC3-positive squamous cell lung carcinoma as first-line therapy. Due to its broad applicability across multiple indications and favorable first-line positioning, BGB-B2033 is expected to capture approximately 40% of the anti-GPC3 market by 2040 in the seven major markets.
Trispecific T-Cell Engagers
AZD9793, developed by AstraZeneca, represents a first-in-class trispecific IgG1 monoclonal antibody engineered as a CD8-directed T-cell engager (TCE). This innovative molecule incorporates three functional binding domains: dual GPC3 binding sites for enhanced tumor targeting, CD8 bias to selectively activate CD8+ cytotoxic T lymphocytes while minimizing CD4+ T cell engagement, and low-affinity T-cell receptor interaction. This design philosophy aims to achieve precise T-cell-mediated tumor killing while reducing the likelihood of off-target cytokine release. In June 2025, AstraZeneca presented data at ASCO 2025 from the RHEA-1 first-in-human study of AZD9793, highlighting the clinical translation of this innovative platform.
Engineered Cell Therapies: CAR-T and Enhanced Variants
Chimeric Antigen Receptor (CAR) T-cell therapy represents one of the most transformative approaches in cancer immunotherapy. CAR-T cells are autologous T cells that have been genetically modified to express synthetic receptors, which recognize GPC3-positive tumor cells, thereby enabling specific cytotoxic killing without MHC restriction.
ECT204 (ARTEMIS AbTCR-T)
Developed by Eureka Therapeutics, ECT204 leverages the proprietary ARTEMIS platform to enhance CAR-T cell functionality in the hostile solid tumor microenvironment. In preclinical HepG2 xenograft models, ECT204 demonstrated abundant tumor infiltration, significantly surpassing that of conventional GPC3-targeting CAR-T cells. Circulating ECT204 T cells exhibited markedly lower PD-1 expression compared to CAR-T counterparts, indicating reduced T-cell exhaustion—a major barrier to CAR-T efficacy in solid tumors. ECT204 received FDA Orphan Drug Designation in 2022 and is currently in Phase II development for unresectable, recurrent, and/or metastatic HCC.
BOXR1030 (Metabolically Enhanced CAR-T)
Developed by SOTIO Biotech, BOXR1030 represents an innovative approach to overcoming challenges in the tumor microenvironment. This autologous T-cell therapy co-expresses a GPC3-targeting CAR and Glutamic-oxaloacetic Transaminase 2 (GOT2), a mitochondrial enzyme that enhances T-cell functionality by improving mitochondrial function. By addressing the metabolic challenges that CAR-T cells face in solid tumors, BOXR1030 aims to sustain anti-tumor activity and resilience in hostile microenvironment conditions. In June 2024, SOTIO Biotech presented Phase I/II DUET-01 trial-in-progress data at the 2024 ASCO Annual Meeting, demonstrating the clinical feasibility of this metabolically enhanced approach. BOXR1030 is positioned to capture approximately 35% of the anti-GPC3 market by 2040 in the seven major markets.
Targeted Radiopharmaceuticals: Precision Radiation Therapy
Targeted radiopharmaceuticals represent a sophisticated approach combining molecular targeting with potent radiation therapy. These agents deliver ionizing radiation directly to GPC3-expressing tumor cells while sparing normal tissues, enabling high local tumor control with minimal systemic toxicity.
RYZ801/RYZ811 (Theranostic Pair)
Developed by RayzeBio (a Bristol Myers Squibb company), the RYZ801/RYZ811 pair exemplifies the theranostic approach—combining diagnostic imaging with therapeutic intervention. RYZ801 utilizes Gallium-68 for PET imaging to identify GPC3-expressing tumors, while RYZ811 employs Actinium-225 for therapeutic delivery. This combination enables patient stratification through baseline imaging, real-time treatment monitoring, tailored dosing strategies, and reduced off-target toxicity. In January 2025, PeptiDream announced that RayzeBio had initiated a Phase I/Ib clinical trial of RYZ801 and RYZ811. The theranostic approach represents a significant opportunity for precision medicine, potentially improving clinical outcomes through individualized patient selection and dosing optimization.
225Ac-GPC3 (BAY 3547926)
Bayer’s targeted alpha radiopharmaceutical represents another important advancement in this space. This investigational therapy comprises a high-affinity GPC3-specific antibody conjugated to Actinium-225, an alpha-emitting radionuclide with a 9.9-day half-life. Actinium-225 emits highly energetic alpha particles with a short pathlength (approximately 50-100 micrometers), enabling delivery of potent cytotoxic radiation with minimal bystander damage. The targeted delivery of alpha radiation to GPC3-positive cancer cells induces irreparable DNA double-strand breaks, potentially decreasing cell viability and exerting profound anti-tumor effects. In April 2025, Bayer initiated a Phase I clinical trial of 225Ac-GPC3, marking the first investigational targeted radiopharmaceutical for HCC in Bayer’s portfolio. Preclinical data presented at the AACR Annual Meeting demonstrated low uptake and rapid clearance from normal tissues, along with tumor regression in in vivo models.
Recent Developments in Anti-GPC3 Targeted Therapies Space
- In July 2025, Zymeworks announced FDA clearance of the Investigational New Drug application for ZW251, enabling initiation of clinical trials.
- In June 2025, AstraZeneca showcased Phase I/II RHEA-1 data for AZD9793 at ASCO 2025, highlighting the first-in-human clinical experience with a CD8-guided T-cell engager.
- In April 2025, Bayer initiated the Phase I clinical trial for 225Ac-GPC3 (BAY 3547926), advancing targeted alpha therapy into clinical evaluation.
- In January 2025, RayzeBio (Bristol Myers Squibb) initiated Phase I/Ib clinical trials for RYZ801 and RYZ811, establishing the clinical foundation for theranostic GPC3 targeting.
Anti-GPC3 Targeted Therapies: What Lies Ahead?
Anti-GPC3 targeted therapies represent a transformative frontier in cancer treatment, addressing a significant unmet need in hepatocellular carcinoma and select solid tumors. The evolution from early monoclonal antibodies toward sophisticated next-generation platforms demonstrates the field’s commitment to overcoming historical limitations and delivering clinical benefit to patients.
The total market for anti-GPC3 targeted therapies across the 7MM is expected to grow significantly, rising from approximately USD 24 million in 2032 to around USD 3.2 billion by 2040, as per DelveInsight. This sharp expansion is driven by the accelerating clinical adoption of next-generation GPC3 therapies, a growing prevalence of GPC3-expressing cancers, and the anticipated launch of first-in-class treatments.
The current pipeline includes multiple promising programs advancing through clinical development, with BGB-B2033 and BOXR1030 positioned as potential market leaders based on their distinct therapeutic approaches and broad indication potential. The achievement of clinical efficacy breakthroughs with these programs could reshape treatment paradigms for advanced HCC and establish GPC3 as a validated therapeutic target comparable to other successful precision oncology approaches.
Critical success factors for the anti-GPC3 field include rigorous patient selection through biomarker-driven strategies, rational combination approaches to overcome microenvironmental barriers, and systematic investigation of dosing optimization and safety management. As clinical development programs mature and provide real-world efficacy and safety data, anti-GPC3 targeted therapies are poised to fulfill their significant therapeutic promise and deliver meaningful improvements in survival for patients with previously difficult-to-treat malignancies.

Downloads
Article in PDF
Recent Articles
- Seagen/Astellas plans to expand their Antibody Drug Conjugate (ADC), Padcev in additional bladder...
- Nanoscope’s MCO-010 Begins FDA Rolling Submission for Retinitis Pigmentosa; Bayer’s KERENDIA Appr...
- Agios’ cancer pipeline auction; uniQure’s gene therapy on hold; Ultragenyx, Mereo Deal; aTy...
- 5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
- Bispecific and Trispecific Antibodies: Are They Better Than CAR-Ts?