What Does the Future of Plaque Psoriasis Treatment Market Look Like?
Apr 07, 2023
Table of Contents
Psoriasis is a common skin condition that accelerates the life cycle of skin cells. More than 3% of the adult population in the United States suffers from psoriasis. That equates to more than 7.5 million US adults. According to the World Psoriasis Day Consortium, 125 million people worldwide (2-3% of the total population) have psoriasis. According to the National Psoriasis Foundation (NPF), more than 8 million Americans suffer from psoriasis. As per Delveinsight’s estimates, the total prevalent population of psoriasis in the seven major markets was around 16 million cases in 2021. Plaque psoriasis is the most common type of psoriasis, affecting 80% of people with the disease. According to the latest published “Plaque Psoriasis Epidemiology” report, it was found that males are slightly more affected by plaque psoriasis than females.
The Current Plaque Psoriasis Treatment Space
There is currently no cure for psoriasis, but plaque psoriasis treatment can slow the growth of skin cells and relieve pain and itching. The severity of the disease determines the type of plaque psoriasis treatment. Phototherapy alone or in combination with systemic agents is generally recommended for moderate to severe plaque psoriasis treatment. The first line of active plaque psoriasis treatments includes the use of topical agents such as moisturizers, vitamin D creams, topical retinoids, and medications such as TAZORAC®, Duobrii (Ortho Dermatologics), and coal tar. TAZORAC (Allergan, Inc) Gel, 0.05%, and 0.1% are indicated for the topical plaque psoriasis treatment with up to 20% body surface area involvement.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Evaluating the Upcoming Drugs in Pipeline for Major Autoimmune Diseases
- December 7 Business Cocktail
- Plaque psoriasis drug outshines in phase III trial; Immunovant announces pause in trial of IMVT-1...
- Bristol-Myers Squibb’s Opdivo & Yervoy Combo Trial; Sarepta’s Gene Therapy SRP-9001 for DMD;...
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
It was approved by the FDA in 1997 and is widely used as a topical agent for plaque psoriasis treatment. Duobrii (Ortho Dermatologics) is a combination of the corticosteroid halobetasol propionate and the retinoid prodrug tazarotene. It was approved by the FDA in 2019 and is specifically indicated for topical plaque psoriasis treatment in adults. Phototherapy, oral-systemic agents, and injectable biological therapies are recommended when topical therapy fails. Phototherapy is a mainstay moderate to severe psoriasis treatment, particularly when topical agents are ineffective. It comes in psoralen plus UVA, broadband UVB, and narrowband UVB formulations (NB-UVB). As demonstrated in multiple RCTs, NB-UVB therapy is frequently used as a first-line plaque psoriasis treatment due to its efficacy and safety benefits.
Systemic plaque psoriasis treatments are frequently used in conjunction with topical therapy and phototherapy for patients with severe psoriasis. Oral agents and injectable biological therapies are currently available as systemic plaque psoriasis treatments. Among the FDA-approved drugs for plaque psoriasis treatment are Amevive (Biogen IDEC), Cimzia (UCB), Cosentyx (Novartis), Ilumya (Sun Pharmaceuticals), Otezla (Celgene), Skyrizi (AbbVie), and many others.

Plaque Psoriasis Treatment: Robust Pipeline
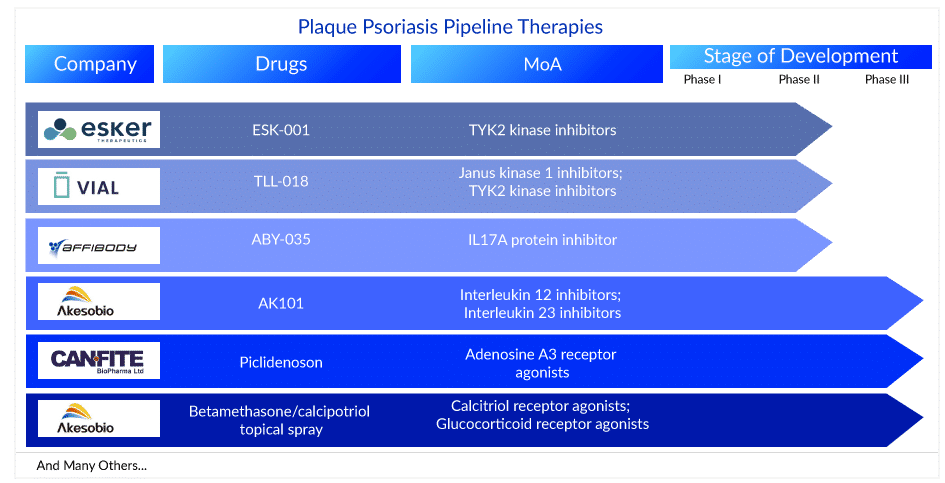
The plaque psoriasis pipeline is very robust as 75+ key players are involved in developing therapies for the management and plaque psoriasis treatment. These plaque psoriasis medications are currently in different stages of development for plaque psoriasis treatment. AK101 (Ebdarokimab) (Akeso Biopharma), CF101 (Can-Fite Biopharma), and Betamethasone/calcipotriol topical spray (Lipidor) are in the late stages (Phase III) of development for plaque psoriasis treatment. Whereas TAK-279 (Takeda), TLL-018 (Vial), ESK-001 (Alumis), M1095 ((Avillion LLP), ABY-035 (Affibody), JNJ-77242113 (Janssen Biotech), ME3183 (Meiji Seika Pharma), and ADX-629 (Aldeyra Therapeutics) are currently in the Phase II stage of development for plaque psoriasis treatment. The other plaque psoriasis drugs in the pipeline include DLX105-DMP (Delarrivo), GLPG3667 (Galapagos NV), ONO-4685 (Ono Pharmaceutical), CT303 (GC Cell), AT193 (Azora Therapeutics), and others. The expected launch of these plaque psoriasis treatment drugs might fuel the market in the coming years.
AK101: Akeso Biopharma
AK101 injection is a novel antibody drug developed independently by Akeso Biopharma under the National Major Innovative Drug Projects. It is intended for the treatment of autoimmune diseases such as psoriasis, Crohn’s disease, ulcerative colitis, and lupus. AK101 clinically treats autoimmune diseases by inhibiting the biological activities of the cytokines IL-12 and IL-23. Johnson & Johnson’s Ustekinumab (trade name: Stelara), which has the same drug targets, has been approved by the FDA for psoriasis treatment, Crohn’s disease, and ulcerative colitis and generated US$6.4 billion in sales in 2019. It is currently in Phase III of a clinical trial for plaque psoriasis treatment.
Betamethasone/calcipotriol topical spray: Lipidor
AKP02 is a psoriasis drug candidate based on Lipidor’s patented AKVANO technology that combines calcipotriol and betamethasone. The goal of AKP02 is to provide a simple, spray-based mild to moderate plaque psoriasis treatment. The first patients were enrolled in Phase III clinical study of AKP02 cutaneous spray (calcipotriol + betamethasone dipropionate 0.005%/0.05%) against mild to moderate psoriasis in January 2022.
CF101: Can-Fite Biopharma
Piclidenoson is a novel, first-in-class A3 adenosine receptor (A3AR) small molecule, orally bioavailable drug with an excellent safety profile and efficacy evidence in Phase II clinical studies. The drug’s mechanism of action involves the inhibition of the inflammatory cytokines interleukin 17 and 23 (IL-17 and IL-23) and the induction of apoptosis in patients’ skin cell keratinocytes involved in disease pathogenicity. On 29 June 2022, Can-Fite BioPharma Ltd. announced positive top-line results from the COMFORT trial, a Phase III, multicenter, randomized, placebo- and active-controlled, double-blind study in more than 400 adults with moderate to severe plaque psoriasis. Piclidenoson had an excellent safety profile that matched that of the placebo-treated patients, and it was safer than Otezla. Can-Fite has five out-licensing agreements for Piclidenoson for psoriasis treatment in markets such as Canada, Eastern Europe, Central Europe (Austria, Switzerland, and Spain), China, and South Korea.
TAK-279: Takeda
TAK-279, developed by Takeda, is a late-stage, highly selective oral, allosteric tyrosine kinase 2 (TYK2) inhibitor that is being studied for the treatment of various autoimmune diseases. TAK-279 has shown excellent functional selectivity and broad therapeutic margins in preclinical studies. TAK-279 demonstrated a good tolerability profile, a dose-dependent trend in exploratory clinical activity, and a pharmacokinetic profile that allows for once-daily solid oral dosing in Phase I studies. TAK-279 is currently being studied in Phase IIb trial for active psoriatic arthritis. TAK-279 is a research compound that has not been approved for use by any regulatory body. Recently, Takeda announced positive results from a Phase IIb plaque psoriasis clinical trial of TAK-279. The study met its primary and secondary endpoints, with a statistically significant higher proportion of TAK-279 patients achieving Psoriasis Area and Severity Index (PASI) 75, 90, and 100 in the 5mg, 15mg, and 30mg dosing arms compared to placebo at 12 weeks.
TLL-018: Vial
TLL-018 is a novel TYK2/JAK1 inhibitor that is both potent and selective. The key regulators of pro-inflammatory cytokines are TYK2 and JAK1. Patient screening and enrollment are currently underway, with the first patient enrolled and receiving the first dose. The screening was completed in 6 days by the Integrative Skin Science and Research site team in Sacramento, CA, led by Dr. Raja Sivamani, principal investigator. Integrative Skin Science and Research is a full-service adult and pediatric medical, surgical, and cosmetic dermatology practice specializing in integrative skin health and wellness and cutting-edge clinical trial research. Recently, on 19 January 2023, Vial reported that the first patient had been enrolled in the clinical trial TLL018-205, a Phase II, randomized, double-blind, placebo-controlled research to assess the efficacy and safety of TLL-018 in human patients with moderate to severe plaque psoriasis. Hangzhou Highlightll Pharmaceutical Co., Ltd, a pharmaceutical company focused on drug development for immune and inflammatory diseases, is funding this research.
ABY-035: Affibody
ABY-035, developed by Affibody, is a novel bispecific agent that effectively targets both IL-17A subunits and albumin. ABY-035 was specifically designed to take advantage of the strengths of Affibody’s technology platform to create a very small protein drug (18 kDa, one-eighth the size of an antibody) with extremely high apparent affinity to IL-17A (KD 300fM) and antibody-like half-life due to the strong (KD 50pM) binding affinity to serum albumin. Currently, it is being evaluated in the Phase II stage of development for plaque psoriasis treatment.
Major Clinical Developments and Breakthroughs in Recent Times in the Plaque Psoriasis Treatment Domain
- In March 2023, Glenmark Pharmaceuticals announced that it had received approval from the US Food and Drug Administration to market a generic medication for plaque psoriasis treatment.
- In March 2023, Bristol Myers Squibb announced that the European Commission had approved Sotyktu (deucravacitinib) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
- In March 2023, Takeda announced positive results from a Phase IIb plaque psoriasis clinical trial of TAK-279 (NDI-034858), a highly selective, oral, allosteric tyrosine kinase 2 (TYK2) inhibitor, in patients with moderate-to-severe plaque psoriasis.
- In January 2023, Vial, a global tech-enabled CRO providing next-generation psclinical trial management services, announced the enrollment of the first patient in the clinical trial TLL018-205, a Phase II, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of TLL-018 in human subjects with moderate to severe plaque psoriasis.
- In December 2022, UCB, a global biopharmaceutical company, announced that the FDA has accepted for review the Biologics License Application (BLA) resubmission for bimekizumab for moderate to severe plaque psoriasis treatment in adults. The FDA classified the resubmission as ‘Class 2’ with a six-month review period. The FDA action is expected in the second quarter of 2023.
- In December 2022, Arcutis Biotherapeutics, Inc. announced the submission to the U.S. Food and Drug Administration (FDA) of a supplemental new drug application (sNDA) for the expanded indication of ZORYVE (roflumilast) cream 0.3% for plaque psoriasis treatment in children ages 2 to 11.
- In November 2022, Dermavant Sciences released its first commercial as part of a national, multi-platform, direct-to-consumer marketing campaign for VTAMA® (tapinarof) cream, 1%.
- In September 2022, Alumis Inc. announced that the first patient had been dosed in Stride, a Phase II clinical trial of ESK-001 for the treatment of patients with moderate to severe plaque psoriasis.
- In May 2022, Bristol Myers Squibb announced two-year results from the POETYK PSO long-term extension (LTE) trial demonstrating durable efficacy and a consistent safety profile with deucravacitinib treatment in adult patients with moderate to severe plaque psoriasis. Clinical efficacy was maintained through up to two years of deucravacitinib treatment, with response rates at Week 60 in the LTE of 77.7% and 58.7% for Psoriasis Area and Severity Index (PASI) 75 and static Physicians Global Assessment (sPGA) 0/1 (clear/almost clear skin), respectively.
- In May 2022, Nimbus Therapeutics announced the presentation of new data from multiple Phase I clinical studies of its investigational oral, allosteric tyrosine kinase 2 (TYK2) inhibitor, NDI-034858, in both healthy volunteers and patients with moderate-to-severe psoriasis.
- In April 2022, Amgen announced preliminary results from a Phase III study evaluating the efficacy and safety of ABP 654 compared to STELARA® (ustekinumab) in adult patients with moderate to severe plaque psoriasis. The study met the primary efficacy endpoint, demonstrating no clinically meaningful differences between ABP 654 and STELARA.
- In March 2022, Arcutis Biotherapeutics announced new pooled results from the DERMIS Phase III trials of roflumilast cream are available in four abstracts at the 2022 American Academy of Dermatology annual meeting that took place in Boston, MA. Roflumilast is a selective and highly potent phosphodiesterase-4 inhibitor (PDE4) being investigated by Arcutis as a non-steroidal topical treatment for multiple inflammatory skin diseases, including plaque psoriasis.
Future Directions in the Plaque Psoriasis Treatment
The history of psoriasis treatment over the last century celebrates the advances made in understanding and caring for patients with this disease. This progress has been made possible thanks to the collaboration of clinicians, patients, academics, and the pharmaceutical industry. While a cure for psoriasis is unlikely in the near future, complete eradication of the disease with the most recent biologic therapies is now a realistic goal for some. A whole-person approach to disease management that incorporates the P4 medicine principles of prediction, prevention, individualized therapy, and patient participation is a logical extension of our understanding that psoriasis is a “systemic disease” with significant physical and psychosocial consequences.
Although systemic psoriasis therapy has advanced significantly, there is still an unmet need for more effective topical therapies and wider use of biosimilars. The use of integrated multi-omic data to stratify patients and guide therapy will be the focus of future research. Improved access to care and early intervention with systemic therapy are topics that are being debated more and more. This is where service development and research intersect, necessitating innovative approaches and research methodologies to achieve this goal.
FAQs
Psoriasis is the most common immune-mediated inflammatory disease, affecting the skin and joints and linked with other systems’ abnormalities. The most common type of psoriasis, plaque psoriasis, causes dry, itchy, raised skin patches (plaques) coated in scales.
Psoriasis is distinguished by small scaly, red bumps. These bumps usually join together to form elevated skin plaques and are most visible on the elbows, knees, and scalp, though any skin area can be affected. The most common plaque psoriasis symptoms are itchiness, skin pain, joint pain, cracked, dry skin, and others.
As psoriasis can resemble eczema and other skin conditions, the plaque psoriasis diagnosis can be difficult. The doctor may need to do a biopsy in some circumstances.
Plaque psoriasis treatment modalities are selected based on disease severity, relevant comorbidities, patient preference (including cost and convenience), efficacy, and individual patient response evaluation. Although there is no cure for psoriasis, numerous effective plaque psoriasis treatment options exist, with topical therapy being the standard of care.

Downloads
Article in PDF
Recent Articles
- AbbVie Announces Results of Study Evaluating SKYRIZI; FDA Fast Track Designation to Arrowhead’s A...
- Bristol-Myers Squibb’s Opdivo & Yervoy Combo Trial; Sarepta’s Gene Therapy SRP-9001 for DMD;...
- World Psoriasis Day
- Biogen-Eisai’s Aduhelm; Quidel acquires Ortho Clinical Diagnostics; Accutar Biotechnology’s...
- Plaque psoriasis drug outshines in phase III trial; Immunovant announces pause in trial of IMVT-1...