The Spinal Cord Injury Treatment Landscape: Limited Progress, Immense Potential
Jun 27, 2025
Table of Contents
Every year, thousands of lives are changed in an instant by spinal cord injuries—yet most people know surprisingly little about them. Spinal cord injury refers to the abrupt damage to the spinal cord’s nerve tissue resulting from trauma, illness, or degenerative conditions. The impact can differ, often leading to upper or lower motor neuron damage and resulting in disruptions to motor, sensory, and autonomic functions, which can be either short-term or lasting.
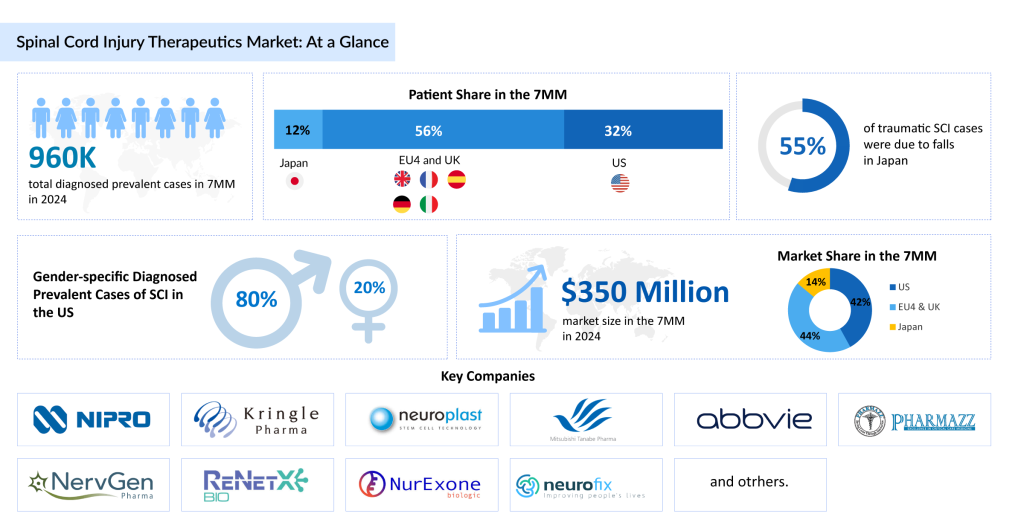
According to DelveInsight, there were approximately 45,000 incident cases of spinal cord injury and around 960,000 diagnosed prevalent cases across the 7MM in 2024. These figures are expected to increase by 2034. The United States accounted for the highest number of diagnosed prevalent SCI cases among the 7MM in 2024, as per DelveInsight’s estimates.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- Next-Gen Spinal Cord Injury Therapies: A Look at the Evolving Drug Pipeline
- MEDIT’s i700 Intraoral Scanner; Phantom Neuro and Blackrock’s Next-Generation Assistive Devices; ...
- Gene and Cell Therapies in CNS Disorders: Miracle Cure? Opportunities Galore!
- Navigating the Emerging Trends and Technologies in the Spinal Cord Injury Rehabilitation Segment
Management of spinal cord injury focuses on stabilizing the injury, supporting functional recovery, and improving outcomes through a combination of medical treatment, surgical intervention, and rehabilitation. Core objectives include controlling pain, enhancing mobility, and fostering patient independence.
However, the absence of curative therapies and the reliance on symptomatic treatment limit long-term progress. Moreover, current clinical practices lack real-time monitoring of the spinal cord, raising the risk of undetected complications that can worsen the condition during the acute phase.
STEMIRAC Stands Alone: The Only Approved SCI Treatment in Japan
The treatment approach for spinal cord injury primarily focuses on rapid medical intervention, surgery, and rehabilitation. In the initial or acute stage, surgical decompression is commonly performed to relieve pressure on the spinal cord, often by removing bone fragments or herniated discs.
Pharmacological therapies also play a key role. Intravenous methylprednisolone may be administered soon after injury to minimize inflammation and nerve damage, although its widespread use remains controversial due to potential adverse effects. Other drugs with anti-inflammatory or neuroprotective properties are employed to mitigate secondary damage.
Rehabilitation involves physical therapy to restore mobility and strength, along with the use of assistive technologies such as wheelchairs and braces to support everyday activities. Chronic pain is managed through medications like tramadol, gabapentin, and cannabinoids. However, a definitive cure for SCI has yet to be found.
Currently, LYRICA (pregabalin) is the only medication approved by the US FDA for neuropathic pain linked to SCI, and generic alternatives are available. Another spinal cord injury treatment is STEMIRAC, an autologous mesenchymal stem cell therapy developed by Nipro Corporation. It involves extracting, culturing, and cryopreserving the patient’s own bone marrow-derived stem cells, which are later infused intravenously. This therapy supports tissue repair, reduces inflammation, and aids recovery through immunomodulatory effects and secretion of supportive factors.
STEMIRAC received conditional approval in 2018 from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) for the treatment of neurological symptoms following traumatic SCI. This approval came with a seven-year post-marketing surveillance requirement to confirm long-term safety and effectiveness. Ongoing efforts aim to accelerate its full-scale clinical implementation, with full approval anticipated by December 2025.
In July 2023, Nipro Corporation launched a clinical trial targeting chronic SCI, aiming to expand STEMIRAC’s use beyond the subacute phase. This move is intended to speed up commercialization and make the therapy more accessible to a broader patient population.
SCI Treatment Space Heats Up with Growing Pipeline Activity
Currently, there are limited approved therapies specifically for spinal cord injury. To overcome this unmet need in the spinal cord injury treatment space, several companies are advancing their assets through various stages of development, driving innovation in the SCI treatment market. Their efforts are shaping a dynamic landscape and opening up significant opportunities for market growth and expansion.
The SCI treatment pipeline continues to expand, featuring candidates such as Kringle Pharma’s KP-100IT, Neuroplast’s Neuro-Cells, Mitsubishi Tanabe Pharma America’s MT-3921, AbbVie’s Elezanumab, Pharmazz’s PMZ-1620, Neurofix’s NFX88, NervGen Pharma’s NVG-291, ReNetX Bio’s AXER-204, and NurExone’s ExoPTEN, among others.
Kringle Pharma (KP-100IT), an innovative therapy in advanced development, is in Phase III clinical trials and is anticipated to launch by 2025 in Japan for the treatment of SCI.
The anticipated launch of these emerging therapies for SCI treatment are poised to transform the market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the SCI treatment market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Recent Developments in Spinal Cord Injury Treatment Space
- In June 2025, āshibio announced that the first patient had been dosed in its Phase 1b clinical trial of andecaliximab for spinal cord injury (SCI). The trial, named ANDECA-HO, is focused on assessing patients at risk of developing heterotopic ossification (HO)—a disorder marked by abnormal bone growth in muscles, tendons, ligaments, and other soft tissues.
- In June 2025, Kringle Pharma, Inc. announced that the FDA had granted orphan drug designation (ODD) to KP-100IT, its intrathecal formulation of recombinant human hepatocyte growth factor (HGF), for treating the acute phase of spinal cord injury.
- In June 2025, NervGen Pharma Corp. announced positive topline results from the chronic cohort (1–10 years post-injury) of its Phase 1b/2a clinical trial evaluating its lead candidate, NVG-291, for the treatment of spinal cord injury. NVG-291 achieved one of its co-primary endpoints and showed encouraging improvements in the GRASSP score, a tool specifically developed to measure hand function in people with cervical spinal cord injuries.
- In March 2025, NurExone Biologic Inc. announced the successful completion of a key preclinical study supporting its upcoming Investigational New Drug (IND) application. This milestone brings the company closer to initiating first-in-human trials. The study showed that ExoPTEN treatment, across various dosing regimens, led to motor function recovery and notably enhanced blood flow at the spinal cord injury site—an essential element for tissue repair and functional improvement.
SCI Treatment Market to Maintain Upward Momentum Through 2034
Advances in imaging technologies such as MRI, along with standardized assessment tools like the ASIA classification system, have enhanced the precision of spinal cord injury diagnosis and treatment planning; however, current clinical practices still lack real-time spinal cord monitoring, increasing the risk of undetected deterioration during the acute phase.
Meanwhile, the scarcity of approved therapies highlights a significant market opportunity for innovative drugs, particularly as breakthroughs in regenerative medicine, such as stem cell therapy and gene editing, gain momentum. Additionally, the growing emphasis on neurorehabilitation and neuroplasticity opens avenues for developing more effective approaches to improve functional recovery in SCI patients.
DelveInsight estimates that the market size for SCI in the 7MM is expected to grow from USD 350 million in 2024 with a significant CAGR by 2034. This growth is mainly driven by the rising prevalence of SCI, driven by increasing awareness and risk factors like traumatic injuries, aging populations, and advancements in diagnostic tools.
However, the variability in SCI severity and location, the complexity of long-term rehabilitation regimens affecting patient adherence, and the high costs of therapy development, including research, clinical trials, and regulatory compliance, collectively complicate SCI treatment protocols, hinder standardization, reduce therapy effectiveness, and pose significant financial challenges, particularly for smaller companies, ultimately limiting spinal cord injury treatment market growth.

Downloads
Article in PDF
Recent Articles
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- MEDIT’s i700 Intraoral Scanner; Phantom Neuro and Blackrock’s Next-Generation Assistive Devices; ...
- Gene and Cell Therapies in CNS Disorders: Miracle Cure? Opportunities Galore!
- Next-Gen Spinal Cord Injury Therapies: A Look at the Evolving Drug Pipeline
- Navigating the Emerging Trends and Technologies in the Spinal Cord Injury Rehabilitation Segment