Gene Therapies for Epidermolysis Bullosa Treatment: The Next Frontier in Dermatology
Mar 25, 2024
Epidermolysis bullosa is a genetic skin condition marked by severe skin sensitivity and the formation of blisters even from minor friction or injury. This disorder arises from genetic changes that impact the proteins crucial for upholding the skin’s structural strength. The degree of severity in epidermolysis bullosa can vary significantly, spanning from mild forms to those posing life-threatening risks, contingent upon the particular subtype.
Blistering that occurs in any type of epidermolysis bullosa can lead to further problems such as infection, sepsis, and even death. Infants with severe forms of this condition are at a higher risk of mortality. In extreme cases like junctional epidermolysis bullosa, death can unfortunately happen within the first or second year after birth.
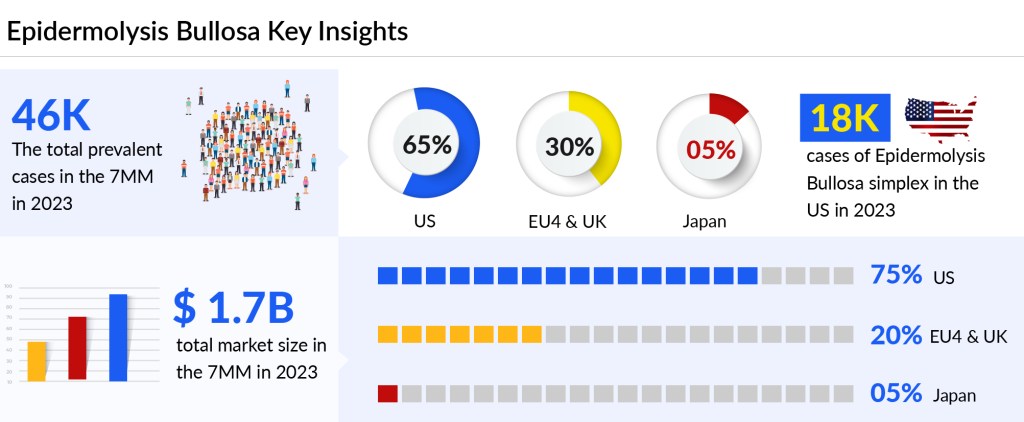
As per DelveInsight analysis, the total prevalent cases of epidermolysis bullosa in the 7MM comprised approximately 46K cases in 2023 and are projected to increase by 2034. The United States contributed to the highest prevalent cases, accounting for ~65% of the 7MM in 2023.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Will The Burgeoning Gene Therapies Make a Difference in Dystrophic Epidermolysis Bullosa Patients...
- Abeona Secures FDA Nod for ZEVASKYN, the First Gene Therapy for RDEB; AbbVie Gains FDA Approval f...
- Gilead’s TRODELVY Shows PFS Benefit in 1L Metastatic TNBC; Otsuka’s Sibeprenlimab Gets FDA Priori...
- FDA Approves RINVOQ for Crohn’s Disease; FDA Approves Krystal Biotech’s Gene Therapy Vyjuve...
- Which Pipeline Therapy Has The Potential To Revolutionize The Dystrophic Epidermolysis Bullosa Tr...
As per the assessments, in the 7MM, the majority of the cases of epidermolysis bullosa are contributed by epidermolysis bullosa simplex followed by dystrophic epidermolysis bullosa and junctional epidermolysis bullosa
Currently, there is no known cure for epidermolysis bullosa. Epidermolysis bullosa treatment strategies revolve around preventing blisters and managing the resulting wounds. The various types of this condition are linked to blister formation in distinct body areas, each with differing levels of severity. Treatments are tailored to the specific type of epidermolysis bullosa.
The main objectives of managing epidermolysis bullosa include shielding the skin from injuries, warding off infections, ensuring optimal nutrition and addressing dietary challenges, reducing deformities and contractures, and fostering a robust support network and a positive mindset. Many of the complications associated with epidermolysis bullosa can be mitigated or prevented through timely and appropriate intervention.
At present, just three epidermolysis bullosa treatments – VYJUVEK (Krystal Biotech), FILSUVEZ (Chiesi Farmaceutici), and JACE (human epidermal cell sheet) (Japan Tissue Engineering) have received approval. Hence, given the current circumstances, there is a notable gap in addressing this disease with effective and precise therapies.
B-VEC is a form of gene therapy that is non-invasive and can be applied topically multiple times. It is developed to deliver two copies of the COL7A1 gene directly to wounds in individuals with DEB. In May 2023, VYJUVEK (beremagene geperpavec-svdt) received approval for the treatment of patients aged 6 months and older with DEB. VYJUVEK marks the first redosable gene therapy ever and is the initial medication sanctioned by the FDA for both recessive and dominant forms of DEB. It can be administered by a healthcare professional either in a clinical setting or at home. The company aims to commence the Marketing Authorization Application process in the EU during the latter part of 2023, with anticipated approval in early 2024. Approval in Japan is anticipated around early 2025.
FILSUVEZ, formerly known as AP101, is an herbal medicinal product featuring birch triterpenes extracted from birch bark. It obtained EU-wide marketing authorization on June 21, 2022. The gel is designated for patients aged 6 months and above, specifically to address superficial wounds linked to Junctional Epidermolysis Bullosa and Dystrophic Epidermolysis Bullosa.
In February 2022, Amryt received a Complete Response Letter (CRL) from the FDA concerning its New Drug Application (NDA) for Oleogel-S10, intended for treating the skin manifestations of dystrophic and Junctional Epidermolysis Bullosa. The FDA requested additional confirmatory evidence of Oleogel-S10’s effectiveness in treating epidermolysis bullosa. Then, in April 2023, Chiesi Farmaceutici finalized the acquisition of Amryt Pharma.
By December 2023, the FDA had granted approval for FILSUVEZ topical gel in treating partial thickness wounds among patients aged 6 months and older diagnosed with Junctional Epidermolysis Bullosa (JEB) and Dystrophic Epidermolysis Bullosa (DEB). FILSUVEZ marks the first approved treatment for wounds associated with JEB.
Despite these approvals, there is still a long way to go in meeting all the needs of epidermolysis bullosa patients, and further research and development are essential to improve treatment options and ultimately enhance the quality of life for individuals living with epidermolysis bullosa worldwide.
Research is currently underway to investigate various treatments for epidermolysis bullosa, including gene therapies (such as gene replacement, gene editing, RNA-based therapy, and natural gene therapy), cell-based therapies (like fibroblasts, bone marrow transplantation, mesenchymal stromal cells, and induced pluripotent stem cells), recombinant protein therapies, as well as small molecule drugs and repurposing existing drugs.
Companies across the globe are diligently working toward the development of novel epidermolysis bullosa treatment therapies with a considerable amount of success over the years. The few candidates in the mid or late stage of investigation for epidermolysis bullosa include EB-101, FCX-007, and RGN-137. The other assets in the early stage of development include TolaSure and AP103. Key players, such as Castle Creek Pharmaceuticals, RegeneRx, Amryt Pharma, Abeona Therapeutics, Krystal Biotech, Shionogi, and BioMendics are developing therapies for the treatment of epidermolysis bullosa.
In the competition to develop gene therapy solutions for epidermolysis bullosa, Krystal has taken the lead by securing FDA approval for VYJUVEK in both recessive and dominant forms of the disorder. In contrast, Abeona and Castle Creek Biosciences have opted for traditional gene therapy methods with their products, EB-101 and D-Fi, respectively, focusing on recessive dystrophic epidermolysis bullosa.
EB-101, an autologous cell therapy, is currently in development for the treatment of recessive Dystrophic epidermolysis bullosa. This treatment approach involves genetically modifying a patient’s skin cells (specifically, keratinocytes and their precursors) with the COL7A1 gene, then reintroducing these cells back into the patient. The goal of EB-101 is to enable the production of normal Type VII collagen and promote the healing of wounds. If successful, EB-101 could become the first approved therapy for RDEB, offering a lasting solution for the challenging issue of large, chronic wounds, which are highly painful and debilitating.
In November 2022, the pivotal Phase III VIITAL study achieved its main goals, showing significant and clinically important advancements in wound healing and pain reduction for individuals with large, chronic Recessive Dystrophic Epidermolysis Bullosa. The company plans to submit its application for EB-101’s approval (BLA) in the third quarter of 2023, with hopes for a prompt launch following potential approval, potentially in the first half of 2024.
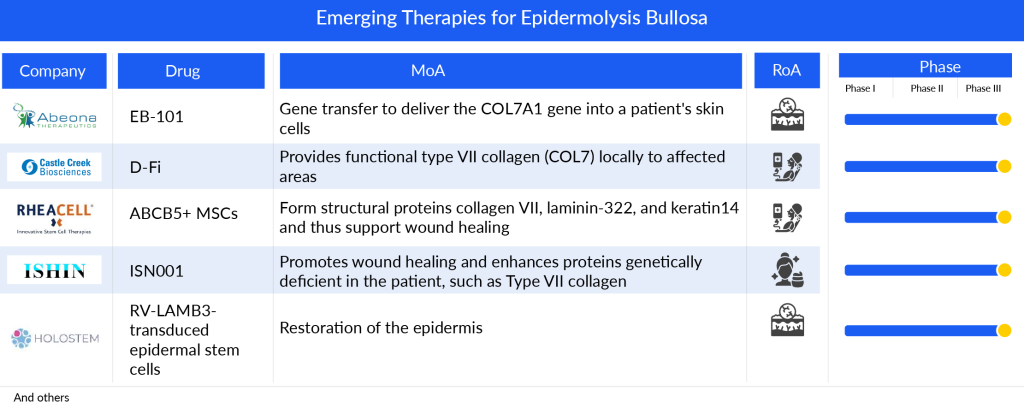
D-Fi, also identified as FCX-007 (dabocemagene autoficel), represents a potential autologous gene therapy for addressing dystrophic Epidermolysis Bullosa (DEB), a rare genetic skin condition marked by progressive, intensely painful, and incapacitating effects. Presently, D-Fi is advancing through Phase III clinical trials aimed at localized treatment for chronic wounds in those affected by recessive dystrophic epidermolysis bullosa. The clinical trial strategy involves a multi-center, randomized, controlled, open-label study conducted on patients, expanding upon findings from the Phase I/II clinical investigation.
Moreover, exclusively in Japan, Shionogi and Ishin Pharma are focusing their development work. Shionogi’s Redasemtide is presently in Phase II trials for DEB, with discussions ongoing with the Pharmaceuticals and Medical Devices Agency (PMDA) for potential drug approval following these trials and subsequent studies.
The anticipated rapid adoption of VYJUVEK in the United States is likely, given that Cheisi’s product recently received a Complete Response Letter (CRL), leaving its US launch uncertain. This situation could potentially provide VYJUVEK with a period of market exclusivity in the US. In contrast, the introduction of Abeona’s EB-101 is expected to have a moderately swift uptake upon its 2024 launch, but its use will be limited to patients with Recessive Dystrophic Epidermolysis Bullosa (RDEB), constituting around 15% of the total epidermolysis bullosa patient population, mainly severe cases.
Abeona and Castle Creek Biosciences are both developing promising therapies targeting this same patient group, setting the stage for intense competition as they are projected to launch around the same time. However, the dynamics are markedly different in Europe, where FILSUVEZ is expected to gain an advantage in terms of rapid adoption. This advantage stems from being the first mover in the market and having a broader patient base included in its European Medicines Agency (EMA) label. Notably, as of July 2023, FILSUVEZ has only been launched in Germany, further highlighting its growth potential.
As per DelveInsight’s estimates, the potential epidermolysis bullosa drugs that can mark a significant change in the forecast period include JACE, FILSUVEZ, VYJUVEK, PTR-01, RV-LAMB3-transduced epidermal stem cells, and others. The total epidermolysis bullosa market size in the 7MM is USD 1.7 billion in 2023 and is projected to increase during the forecast period (2024–2034). Among the emerging therapies, VYJUVEK is expected to cross the USD 1 billion mark and generate the highest revenue in the 7MM epidermolysis bullosa treatment market.
Despite facing difficulties, epidermolysis bullosa specialists and researchers maintain a strong dedication to exploring novel treatment approaches. Delving deeper into this area is vital for patients, given the significant toll epidermolysis bullosa takes on their quality of life and lifespan, especially when complicated by chronic wound infections or sepsis. The determination to discover effective therapies for epidermolysis bullosa endures, underscoring the critical need for ongoing research endeavors.

Downloads
Article in PDF
Recent Articles
- FDA Approves RINVOQ for Crohn’s Disease; FDA Approves Krystal Biotech’s Gene Therapy Vyjuve...
- Pharma Players Line Up To Launch Novel Therapies Fueling Epidermolysis Bullosa Market Size
- Watershed Moment for Cell Therapies and Complicated Journey of Gene Therapies in Japan
- Which Pipeline Therapy Has The Potential To Revolutionize The Dystrophic Epidermolysis Bullosa Tr...
- Cytokinetics Announces Results From SEQUOIA-HCM Clinical Trial of Aficamten; FDA Approves Chiesi’...