Neurovascular Thrombectomy Devices Market
Jul 05, 2021
Table of Contents
Neurovascular thrombectomy devices are used for eliminating the blood clots that have accumulated in the neurovascular vessels during an acute ischemic stroke. These devices comprise a wide range of endovascular tools that are capable of removing thrombi from Neurovasculature in the case of patients suffering from an acute ischemic stroke. These devices are effectively used in the treatment of the blockage of the brain that might arise due to multiple reasons such as atheroma and thrombus within one or more arteries. Stroke is the major cause of disability and most of the cases that require treatment via neurovascular thrombectomy are those of an ischemic stroke. The blood clot present in the arteries can cause fatal complications if left untreated. In developed regions of the world, ischemic stroke is one of the prominent causes of both morbidity and mortality. Rapid and efficient vessel recanalization is an emergent need for the successful treatment of such acute stroke patients.
Devices and Usage
These devices are recommended in various indications. Some of them are entailed below:
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Innovative Solutions, Lifesaving Possibilities: Evaluating the Artificial Organs Market Dynamics
- Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&...
- Pensar Medical’s Partnership with Centurion Therapeutics; BD’ Acquisition of Critical Care; Haleo...
- Revolutionizing Pain Management: The Growth in Innovative Devices and Rise of Market
- Robotic Surgery: Navigating the Growing Demand, Ongoing Trends & Developments in the Global ...
- Stroke and Transient Ischemic Attack
A blood clot or fatty deposits tend to block the artery that is in the process of supplying blood to the brain, thereby causing an ischemic stroke. The ischemic stroke is caused more than 80% times of all the other strokes, the reason being either local thrombosis or embolic vessel occlusion. The blood clots tend to develop due to other conditions such as hypertension, atherosclerosis, and other blood problems. The blood clot formation process is known as thrombosis. A heart attack or a stroke may take place when the clot does not allow the passage of blood to the brain. The microangitopathic strokes accompanied by small infarcts are caused most frequently by local thrombosis, in miniature sized-so called perforator vessels.
- Atherosclerosis: Carotid and Vertebral Artery Disease
When the main blood vessels carry all the oxygenated blood to the brain marrow, it is called carotid and vertebral artery disease or stenosis. Atherosclerosis or “stiffening of the arteries” is the most frequent cause of artery narrowing. Atherosclerosis can be both asymptomatic and symptomatic.
- Brain Aneurysm
A brain aneurysm is an abnormal bulging outward of a vessel wall in one of the brain’s arteries.
- Arteriovenous Malformation (AVM)
An arteriovenous malformation (AVM) is a collection or tangle of abnormal blood vessels that grow in the brain.
- Dural Arteriovenous Fistula/Malformation
A dural AVF/AVM is a collection or tangle of abnormal blood vessels in the dura mater, the thick covering surrounding the brain. It may occur as a result of trauma, infection, or surgery.
Neurovascular Thrombectomy Devices: Types
According to the United States Food and Drug Administration, three diverse classes of mechanical thrombectomy devices have been approved, namely coil retrievers in the year 2004, aspiration devices in the year 2008, and stent retrievers in the year 2012. The available research upon these devices has given enough evidence that mechanical Neurovascular thrombectomy devices are perfectly appropriate for patients with large proximal intracranial artery occlusions due to the emboli arising from the cardiac or arterial region and was found to be most beneficial when performed as soon as feasible after the initiation in patients that are known to be still harboring a salvageable penumbral tissue.
- Coil Retrievers
The coil retrievers comprise a nitinol-shaped memory wire and are delivered via a microcatheter across the targeted clot. After the device is extruded from the delivery catheter, it tends to immediately resume its native coil form. The neurointerventionalist tends to deploy the loops of the coil via the clot to engage the thrombus, pulling back both the coil and the clot back into the catheter. The examples of coil retrievers devices are Merci by Stryker, available in different helix lengths, curvature, diameter, enable its usage in various targeted arteries and the availability of various additional cascading filaments. Other examples include Attractor-18 device (Boston Scientific, Natick, MA), The Catch device (Balt Extrusion, Montmorency, France), and The In-Time device (Boston Scientific, Natick, MA) , among others.
- Aspiration Devices
The suction embedded neurovascular thrombectomy devices comprise vacuum aspirators that are employed in the removal of occlusive clots when the individual is suffering from acute ischemic stroke. Manual aspiration of the targeted thrombi can take place via any microcatheter; the progressing towards suction thrombectomy devices entails a technical solution involving problems associated with the clogging of the suction tips, the most frequent incidence that takes place while application of suction through a small-bore to enable its fitting within the intracranial arteries. The Penumbra system tends to override the problem by attaching a bore separator wire which has a bulbous tip which the operator continuously advances and retracts, thereby disrupting the attached clots and pulling in the thrombus ahead of the catheter.
- Stent Retrievers
The stent retrievers are self-expanding stent which is deployed in the occluded vessel inside the thrombus, pushing it away and entangling it within the stent struts. The stent and the thrombus are therefore withdrawn back within the delivery catheter, the foremost retrievable stent, that was approved in the United States was the Solitaire Flow Restoration Device (Covidien), and various others have been approved in Europe, including Trevo (Stryker), Revive (Codman), MindFrame (MindFrame Inc.), ReStore (Reverse Medical), and Pulse (which combines a stent retriever and an aspiration device, Penumbra). As per Delveinsight’s estimates, the stent-retrivers will occupy more Neurovascular thrombectomy devices market share by device type when compared with aspiration catheters. The rationale behind this was obtained from research studies indicating higher revascularization rates of occluded vessels in less time. The devices had attained more elevated rates of favorable clinical outcomes; however, the cost of these stent retrievers was more when compared to the aspiration catheters.
Advantages
- The use of these devices enables patients to avoid the use of pharmacologic thrombolysis agents, thereby presumably minimizing the risk for intracerebral hemorrhage.
- The treatment of patients with Neurothrombectomy devices for acute ischemic stroke is extended beyond the 3 h window from the onset of symptoms, after which thrombolytics are usually not used.
- Moreover, these devices fragment the thrombus occlusion, expanding the surface area of the clot and allowing thrombolytic agents to be more easily accessed.
- Neurothrombectomy devices can also provide a faster recanalization than thrombolytics and a treatment choice for thrombi that are resistant to fibrinolytic breakdown.
Disadvantages
- The technological difficulties of navigating mechanical instruments through the intracranial circulation, direct damage to the Neurovasculature (including vasospasm, vessel dissection, perforation, or rupture), and fragmentation of thrombi causing distal embolization into previously unaffected vessels and cerebral territories are some of the drawbacks of Neurothrombectomy devices.
- Patients may not be aware of the brain stroke and the need for immediate treatment. The application of intra-arterial thrombectomy is required within the window of stroke. This window for treatment has been extended to a period of 24 h from the last known normalcy – the condition of neurologic baseline readings.
- The Neurovascular thrombectomy procedure is mainly carried out in thrombectomy stroke centers (TSCs), which are around 2,000 globally, and fewer people have access to them.
Eligible Patient Pool
Stroke is considered to be one of the most prominent causes of death and disability across the world. The incidences of stroke have been surging throughout the globe due to the rising numbers of the geriatric population. The patient population that was considered for the calculation of the eligible patient pool is in between 45 to 80 years of age, as the reported incidences of stroke for this age range have been the maximum. As reported in the global burden of diseases study, published by Lancet in 2016, the incidents of cases in the United States were reported to be731,256, 242,497 cases in Germany, 131,416 in France, 166,015 in Italy, 101,845 in Spain, 134,979 in the UK, and 353,551 in Japan. The number of ischemic strokes is calculated from another research published by the Society of Vascular and Interventional Neurology that illustrates, about 30% of the strokes that occur due to large vessel occlusion (LVOs) in the brain comprise of ischemic strokes. Based on this data, the number of large vessel occlusion patients with ischemic strokes are calculated in the United States, EU5, and Japan.
According to DelveInsight’s Neurovascular thrombectomy devices market analysis, the total eligible patient population for Neurovascular Thrombectomy Devices was 30,179 patients in seven major markets in 2018 and is expected to increase at a substantial CAGR by 2026. The largest population that is eligible for neurovascular thrombectomy devices was found in the United States.
Neurovascular thrombectomy Devices in the Pipeline
The stent-retriever thrombectomy and the direct-aspiration thrombectomy are the two most prominent forms of neurovascular thrombectomy. However, combination therapies have improved the rates of successful recanalization in recent years; this combinational technique is called the SOLUMBRA technique, which comprises a combination of large-bore aspiration catheters and stent-retriever technique simultaneously. In the initial clinical trials performed on stroke patients, namely the MR CLEAN, EXTEND-IA, ESCAPE, and SWIFT PRIME, either the ADAPT (direct aspiration technique) or stent-retriever followed by a stent-retriever thrombectomy was used as a recovery option. Kang et al. had illustrated improved rates of recanalization via the switching strategy, with 85.1% of patients achieving thrombolysis in cerebral infarction 2b or 3 recanalizations versus 73.8% without the switching strategy. Additionally, 3-mo functional results were statistically significantly improvised in patients that were treated with the switching strategy as compared to those who were not.
Since the innovation of the switching strategy, the SOLUMBRA technique was more widely adopted. Lee et al., in the year 2013, had illustrated improved recanalization of ICA (internal carotid artery) terminus occlusions with combinational therapies, which was further supported by Humphries et al. in the year 2015 for including combinational therapies from 6-high volume centers. The Humpries et al. validated the use of SOLUMBRA technique, receiving more than 88% thrombolysis in cerebral infarction 2b or 3 recanalization, and 44% favored mRS outcomes at about 90 days.
Although SOLUMBRA has pointed towards the safety and efficacy in terms of recanalization, the research that has focused on the long-term performance when compared with the ADAPT and stent-retriever techniques will still be required. Delveinsight estimates that the Neurovascular thrombectomy devices market size calculated for across 7MM by device type results in the maximum market share captured by the combinational procedure type devices (comprising of SOLONDRA and Solumbra), followed by stent retrievers and lastly, the aspiration catheters, in the year 2026. The reason that the combinational procedures will occupy the maximum Neurovascular thrombectomy devices market is that they combine both aspiration and stent retrievers, and according to the research studies that are ongoing, their efficacy and safety results reported are beyond expectations. In a 2020 research study, the recanalization rates obtained via SOLUMBRA were 86%. The study also claims that it requires very few attempts for the completion of mechanical thrombectomy procedure when compared with ADAPT (A Direct Aspiration First Pass Technique) and SR (Stent Retrievers).
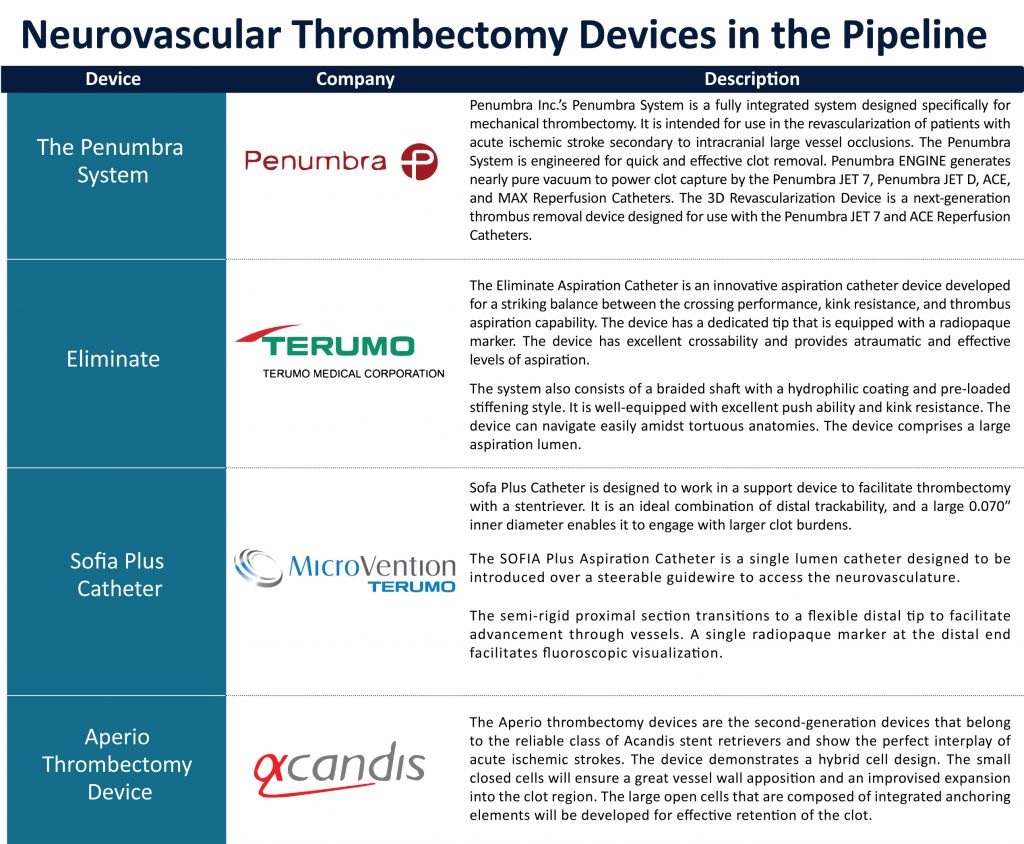
Neurovascular Thrombectomy Devices: In the Spotlight
- On May 06, 2020, the Control Medical Technology had been granted the United States Food and Drug Administration clearance for Aspire MAX 7 – 11F Mechanical Thrombectomy platform for the removal of blood clots from the peripheral vessels.
- On March 10, 2021, Perflow Medical had been granted the launch of Cascade™ 17 Non-Occlusive Remodeling Net in the European market, thereby expanding the Cascade product portfolio.
- On June 02, 2020, Phenox had been granted the launch of NEW pRESET 5-40 & pRESET LUX thrombectomy devices. The devices had received CE Mark approval for these devices in the year 2019.
- On April 30, 2019, Medtronic announced the commercial launch of Solitaire X revascularization device in the United States. In addition, Medtronic also introduced the Phenom 21 catheter to facilitate the delivery of all sizes of the Solitaire X device.
- On February 27, 2020, the CEREBASE™ DA Guide Sheath obtained Food and Drug Administration approval.
Neurovascular Thrombectomy Devices Market
The mechanical thrombectomy devices have brought about a revolution in the treatment of large-vessel occlusion stroke brought about a prominent improvement in patient outcomes. New aspiration catheters and retrievable stents are being developed at a rapid rate. Neurovascular thrombectomy devices have increasingly risen in popularity due to their rising ease of use and effectiveness in people suffering from acute treatment of ischemic stroke due to LVO (left vessel occlusion). However, the broad spectrum use of these devices had alerted the clinicians of their perioperative complications. VWI has been linked to the development of clinical complications that are observed after the usage of stent retrievers. In order to fill the gaps offered by these standalone devices, there are combinational devices that are rising in the domain of neurovascular thrombectomy and have proved to be expanding at a rapid rate.
There have been various studies that are vouching for its safety and effectiveness in patients, and many more are in the process of confirming the results. The combinational therapy involves the use of both the stent retrievers and aspiration catheters; however, their long-term performances are still under validation when compared with the standalone systems.
As per Delveinsight’s assumptions, the Neurovascular thrombectomy devices market is expected to surge in the future due to the emerging techniques comprising of SOLUMBRA and SOLONDRA, among many others. These combinational therapies are expected to dominate the market in comparison to other first-generation or second-generation devices, as witnessed by their safety and efficacy results.
Downloads
Article in PDF
Recent Articles
- Cartessa Aesthetics teams with Classys for Everesse; LogicMark unveils Freedom Alert Max; GE Heal...
- Stryker’s Tornier Shoulder Arthroplasty; Genesis Acquires JC Medical; FDA Clearance to Single-Use...
- Neuspera Medical’s Nuvella System; Abbott Presented the Data for FreeStyle Libre 2 System; ZEISS ...
- Non-Surgical Body Contouring: What’s Fueling Its Growth in Aesthetic Medicine?
- Bausch + Lomb and Heidelberg Engineering Introduced SeeLuma; Oxford Nanopore and bioMérieux Signs...