Parkinson’s Disease: How Close are we to a Cure?
May 27, 2025
Table of Contents
Parkinson’s disease is a complex and progressive neurodegenerative disorder that affects millions worldwide, with its prevalence expected to rise due to aging populations and environmental factors. Characterized by a gradual decline in motor and non-motor functions, the disease significantly impacts the quality of life for patients. Understanding what Parkinson’s disease is involves recognizing its hallmark motor symptoms, such as tremors, rigidity, bradykinesia (slowness of movement), and postural instability, as well as non-motor complications, including depression, cognitive impairment, and autonomic dysfunction.
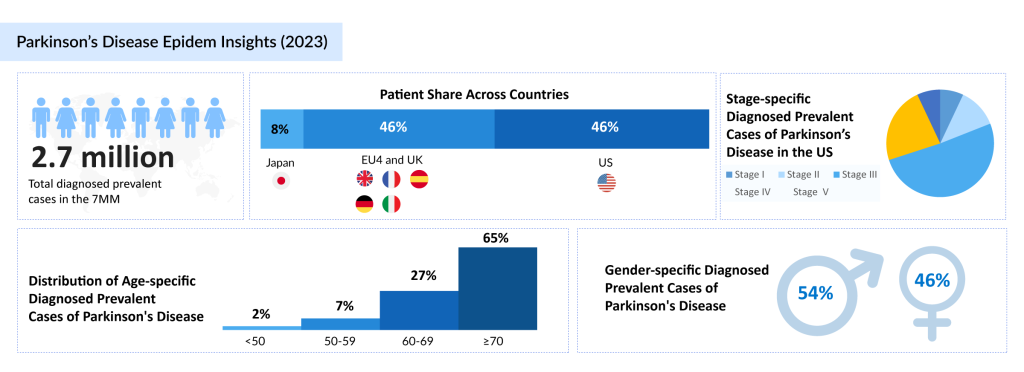
In the 7MM, stage-specific diagnosed prevalent cases of Parkinson’s disease were 11% in Stage I, 28% in Stage II, 35% in Stage III, 20% in Stage IV, and 6% in Stage V. These statistics emphasize the growing impact of the disease and the urgent need for innovative treatments. As the disease progresses through distinct stages of Parkinson’s disease, patients face increasing challenges in managing symptoms, highlighting the need for effective and comprehensive treatments.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- NICE endorsement for Pfizer’s Vizimpro; Eisai, Dundee targets Cancer; Capital raise for AgaMatrix
- Parkinson’s Awareness Month 2019
- Voltage-Dependent T-Type Calcium Channel Blockers
- The Future of Parkinson’s Disease Treatment: Unlocking the Potential of Cell and Gene Therapy
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
In 2024, there were approximately 3 million diagnosed prevalent cases of Parkinson’s disease across the 7MM (the United States, the EU5 countries, and Japan). This growing prevalence underscores the importance of advancing therapeutic solutions to meet the rising demand for symptom management and improved quality of life for individuals affected by the disease.
Current therapies primarily focus on symptom management, with pharmacological options such as levodopa and dopamine agonists forming the cornerstone of care. However, the progression of Parkinson’s disease has driven the demand for advanced therapies that address both motor and non-motor symptoms while potentially modifying the disease’s course.
Emerging research into Parkinson’s disease therapies is focusing on targeting the underlying pathologies, such as alpha-synuclein aggregation and mitochondrial dysfunction, offering new hope for slowing or potentially halting disease progression. Innovations like continuous infusion systems, gene therapies, and brain-penetrant drugs are poised to reshape the treatment landscape, moving beyond symptom management toward more effective, disease-modifying approaches. As we delve into these advancements, we begin to ask: are we closer to a cure? The evolving strategies in Parkinson’s disease treatment are bringing us nearer to that possibility, offering hope to patients and caregivers as they navigate the challenging stages of this condition.
Gain a deeper understanding of Parkinson’s disease by exploring our specially curated blog — click here to dive in and learn more.
Current Treatments and Their Limitations
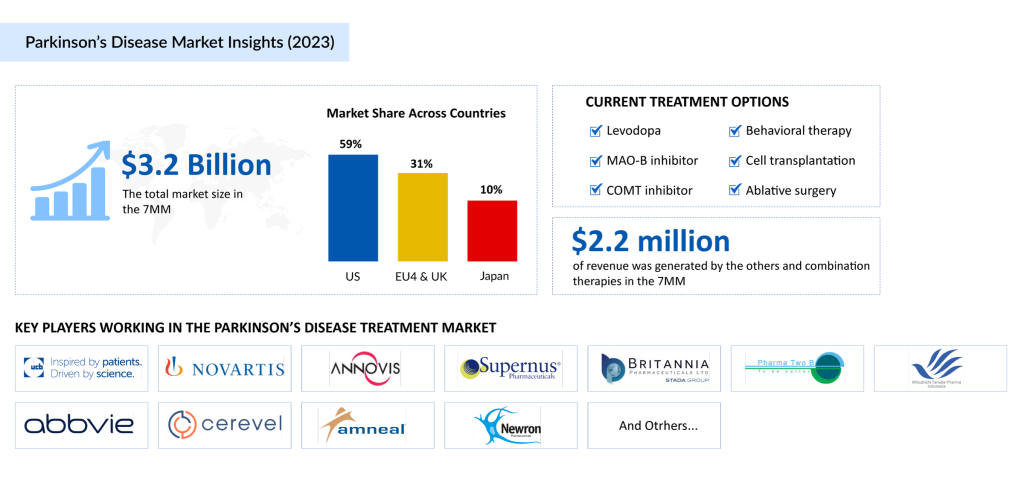
Although the current Parkinson’s Disease treatment landscape lacks a curative option, various pharmacological and nonpharmacological therapies are combined to manage symptoms and improve quality of life. Tailored treatment approaches include physical, occupational, and speech therapy, along with surgical interventions for select patients. Complementary therapies are also increasingly adopted to address specific Parkinson’s disease symptoms and enhance overall care.
Pharmacological Approaches in Parkinson’s Disease Treatment
Multiple medication classes are used in the Parkinson’s Disease therapeutic market, yet carbidopa/levodopa remains the cornerstone of treatment. Known for its high efficacy, it is available in diverse formulations and strengths, allowing for customized therapy across different stages of the disease.
Levodopa, developed over 30 years ago, is widely regarded as the gold standard among Parkinson’s disease therapies. It works by crossing the blood–brain barrier and converting into dopamine, thus replenishing depleted dopamine levels in the brain. While levodopa significantly enhances motor function, common side effects include nausea, vomiting, dizziness, dry mouth, and abnormal involuntary movements (dyskinesia). In some cases, it may lead to confusion, hallucinations, or psychosis.
The combination therapy of carbidopa/levodopa is the most effective treatment for motor symptoms and is often initiated as first-line therapy, especially in patients with late-onset disease. Approved formulations include SINEMET, SINEMET CR, PARCOPA, RYTARY, and DUOPA.
Other Key Drug Classes in Parkinson’s Disease Treatment
- Dopamine Agonists: These agents, such as pramipexole, ropinirole, bromocriptine, and pergolide, mimic dopamine by stimulating dopamine receptors. They are used alone or in combination with levodopa to manage “off” episodes. However, side effects like somnolence, impulsivity, and hallucinations may limit use in some patients.
- COMT Inhibitors: These drugs block catechol-O-methyltransferase, an enzyme that breaks down levodopa. By inhibiting this enzyme, COMT inhibitors increase levodopa’s availability in the brain and enhance its effectiveness.
- Other Therapies: Additional medications commonly used include MAO-B inhibitors, anticholinergics, amantadine, and adenosine A2a antagonists, all contributing to the reduction of motor symptoms.
Several notable drugs have gained approval for Parkinson’s disease treatment in recent years, including PRODUODOPA/VYALEV, CREXONT, XADAGO/EQUFINA, and INBRIJA. The latest addition to the Parkinson’s Disease treatment therapies landscape is ONAPGO, approved in February 2025, signaling continued innovation in the Parkinson’s Disease therapeutic market.
Non-Pharmacologic Approaches
Non-drug therapies play a vital role in the Parkinson’s Disease treatment landscape. These include exercise, physical therapy, occupational therapy, and speech therapy, all of which aim to maintain functional independence and improve quality of life. For patients with medication-resistant symptoms or severe motor complications, advanced interventions like deep brain stimulation (DBS) or levodopa-carbidopa enteral suspension therapy are available. DBS involves implanting electrodes in specific brain regions to regulate abnormal neural activity, providing relief for tremors and reducing dyskinesias.
Limitations of Current Therapies
Despite the diverse array of Parkinson’s Disease therapies, significant unmet needs persist. Most therapies focus on symptomatic relief, leaving the underlying neurodegeneration unaddressed. Motor fluctuations, wearing-off effects, and treatment-related dyskinesias present ongoing challenges in long-term management. Non-motor symptoms, such as depression, cognitive decline, and autonomic disturbances, often remain inadequately managed by existing drugs, further impacting patients’ quality of life.
As the Parkinson’s Disease market evolves, there is a growing demand for innovative treatments that not only improve symptom control but also slow disease progression. The emergence of novel delivery systems, combination therapies, and personalized medicine holds promise for addressing these limitations and reshaping the Parkinson’s Disease therapeutic market.
Recent Advances in Parkinson’s Research
The field of Parkinson’s disease research continues to witness groundbreaking developments, with significant strides being made in both therapeutic innovations and diagnostic technologies. Recent advancements highlight the collaborative efforts of pharmaceutical companies, academic institutions, and biotech firms to address the unmet needs of patients living with Parkinson’s disease.
In April 2025, Cerevance reported topline results from the Phase II ASCEND trial evaluating solengepras as an investigational monotherapy in patients with early-stage, untreated Parkinson’s disease.
In April 2025, Roche presented new data at AD/PD 2025, including the first disclosure of results from the Phase IIb PADOVA study.
In March 2025, MedRhythms announced that MR-005, its neurorehabilitation system designed to support gait rehabilitation and motor function in adults with Parkinson’s disease (PD), has been classified as a Class II, Rx-only medical device by the FDA. The system will be marketed under the brand name Movive.
In March 2025, Medtronic plc, a global leader in healthcare technology, announced the FDA approval of BrainSense™ Adaptive deep brain stimulation (aDBS) and BrainSense™ Electrode Identifier (EI). While there is no cure for debilitating neurological conditions like Parkinson’s, deep brain stimulation (DBS) has been transforming the lives of patients with Parkinson’s and other neurological disorders for over 30 years.
In February 2025, AB-1005, AskBio’s investigational gene therapy for Parkinson’s disease, received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA to accelerate its development and review for treating serious or life-threatening conditions.
In February 2025, Jupiter Neurosciences partnered with Catalent Pharma Solutions to manufacture JOTROL softgel capsules in preparation for its upcoming Phase IIa clinical trial in Parkinson’s disease.
In February 2025, Newronika received FDA Investigational Device Exemption (IDE) clearance to begin a pivotal U.S. clinical trial. The trial will assess the safety and efficacy of its adaptive deep brain stimulation system for patients with movement disorders, including Parkinson’s disease.
In February 2025, Supernus Pharmaceuticals, Inc. announced that the FDA approved ONAPGO (apomorphine hydrochloride) injection, formerly known as SPN-830, as the first and only subcutaneous apomorphine infusion device for treating motor fluctuations in adults with advanced Parkinson’s disease (PD).
In December 2024, AbbVie announced positive topline results from its pivotal Phase III TEMPO-2 trial, evaluating tavapadon as a flexible-dose monotherapy for early-stage Parkinson’s disease. Tavapadon, the first and only D1/D5 partial agonist in its class, demonstrated its potential to reshape the dopamine agonist market by meeting primary and secondary endpoints.
Further expanding the treatment landscape, Cerevance dosed the first patient in its Phase III ARISE trial on November 18, 2024. This pivotal study investigates the efficacy of solengepras, a novel therapy designed as an adjunctive treatment for Parkinson’s patients. By targeting specific cell types, this approach promises a more tailored intervention for managing the disease.
In November 2024, Sunbird Bio unveils promising diagnostic advancements. Their cutting-edge technology successfully classified blood samples from Parkinson’s disease-positive patients with 86% accuracy by directly detecting aggregated alpha-synuclein proteins. This innovation represents a significant step toward early and accurate diagnosis, potentially enabling earlier intervention and better patient outcomes.
In October 2024, AbbVie achieved a milestone with FDA approval for VYALEV (foscarbidopa/foslevodopa), marking a breakthrough as the first 24-hour continuous subcutaneous infusion therapy for advanced Parkinson’s motor fluctuations. This approval underscores a significant leap in drug delivery systems, addressing the challenge of maintaining consistent symptom relief throughout the day.
In September 2024, D&D Pharmatech, a Korean drugmaker, received FDA approval to advance to a Phase II clinical trial of its experimental drug, NLY01, for multiple sclerosis. The decision builds on promising results from earlier trials for Parkinson’s disease. Conducted by researchers at Johns Hopkins University, the study aims to evaluate NLY01’s potential in slowing neurodegeneration in MS, signaling the versatility of this treatment across neurodegenerative conditions.
This period of accelerated innovation reflects the collective drive within the Parkinson’s research community to not only improve symptom management but also target disease progression. Despite challenges, these advancements lay the groundwork for more effective therapies, improved diagnostic tools, and a deeper understanding of Parkinson’s pathology.
Promising Therapies on the Horizon
The landscape of Parkinson’s disease treatment is evolving rapidly, with several promising therapies currently in development. These Parkinson’s disease therapies are designed to target various aspects of the disease, offering hope for improved management of both motor and non-motor symptoms. Below, we highlight some of the most exciting Parkinson’s disease drugs in the pipeline that could redefine Parkinson’s care in the near future.
Cerevel Therapeutics’ Tavapadon
Currently in Phase III trials, tavapadon is a selective dopamine D1/D5 receptor partial agonist designed to provide motor benefits while minimizing common side effects like sedation and psychosis. With its once-daily oral formulation, tavapadon is positioned as a versatile treatment for both early and late-stage Parkinson’s disease. Following AbbVie’s acquisition of Cerevel, tavapadon has gained momentum and is expected to launch in 2025, potentially transforming the dopamine agonist market. Tavapadon’s unique mechanism of action positions it as one of the most promising emerging drugs in Parkinson’s disease therapies.
Pharma Two B’s P2B001
This once-daily combination therapy merges low doses of pramipexole and rasagiline, leveraging their synergistic effects to manage Parkinson’s disease symptoms while minimizing side effects. Phase III data revealed strong efficacy and tolerability, positioning P2B001 as a potential first-line treatment option. An FDA submission is anticipated in 2026. P2B001 is a key player in the Parkinson’s disease pipeline, offering a novel combination approach to treating motor symptoms.
Cerevance’s Solengepras
Targeting the GPR6 receptor, solengepras represents a novel approach to reducing OFF time and mitigating the side effects of traditional dopaminergic therapies. With significant reductions in daily OFF time observed in Phase II trials and ongoing Phase III studies, Solengepras is poised to provide a precision-driven treatment option that enhances patients’ quality of life. Solengepras is a standout in the Parkinson’s disease pipeline, potentially offering a breakthrough in symptom management with fewer side effects.
BioVie’s NE3107
A novel anti-inflammatory and insulin-sensitizing agent, NE3107, is under investigation for its potential to reduce neuroinflammation and improve motor function in Parkinson’s patients. This unique mechanism offers a multi-faceted approach to symptom management, with promising early clinical results. As an emerging drug in the Parkinson’s disease pipeline, NE3107 has the potential to address both neuroinflammation and motor symptoms, which could pave the way for a more comprehensive treatment strategy.
ABL Bio, Inc.’s ABL301
This innovative therapy utilizes a bispecific antibody targeting alpha-synuclein and the IGF1 receptor, aiming to facilitate the delivery of therapeutic agents across the blood-brain barrier. By addressing the accumulation of alpha-synuclein, ABL301 holds promise as a disease-modifying treatment that could slow Parkinson’s progression, positioning it as a potential leader in Parkinson’s disease therapies that target the root cause of the disease.
These Parkinson’s disease therapies, each targeting distinct mechanisms of action, highlight the innovative strides being made in Parkinson’s disease research. As clinical trials progress, these Parkinson’s disease drugs could not only enhance symptom management but also bring us closer to disease-modifying therapies, marking a new era in Parkinson’s disease care. With the pipeline of emerging drugs continuing to expand, the future of Parkinson’s disease treatment looks brighter than ever.
To explore the most promising Parkinson’s disease therapies in detail, check out our comprehensive article on the emerging drugs in the Parkinson’s disease pipeline!
Biomarkers and Early Diagnosis
The early diagnosis of Parkinson’s disease remains a significant challenge due to the lack of definitive biomarkers that can accurately predict its onset and progression. However, the potential for early detection is an exciting area of research, especially with the development of biomarkers that can detect the underlying pathology of the disease before the onset of symptoms. Advances in imaging techniques and the identification of promising biomarkers offer new hope for better diagnostic accuracy, early intervention, and more targeted treatments.
Biomarkers for Parkinson’s disease are crucial not only for early detection but also for monitoring disease progression and assessing treatment responses. These biomarkers can reflect key pathophysiological processes, such as alpha-synuclein aggregation, mitochondrial dysfunction, and neuroinflammation, which are central to PD pathology. While there are several promising biomarkers in development, including blood-based, cerebrospinal fluid (CSF), and imaging markers, no single biomarker has yet proven to be sufficiently reliable for routine clinical use.
The potential for combining multiple biomarkers—such as genetic, protein, and imaging markers—could lead to more accurate and earlier diagnoses, allowing for interventions before significant neurodegeneration occurs. Furthermore, artificial intelligence algorithms are being explored to enhance the identification and interpretation of these biomarkers, accelerating early diagnosis and enabling better patient stratification for clinical trials. AI’s role could also extend to predicting responses to specific therapies, a critical factor in developing personalized, disease-modifying treatments.
The pharmaceutical industry is keenly focused on leveraging biomarkers to develop therapies that not only manage symptoms but target the underlying causes of Parkinson’s disease. By utilizing biomarkers for patient stratification, pharmaceutical companies can better design clinical trials, identify at-risk populations, and create drugs that slow or halt disease progression. The potential for biomarkers to transform the landscape of Parkinson’s disease diagnosis and treatment is immense, paving the way for more effective, individualized care.
With the current lack of curative therapies, biomarkers present a crucial opportunity to move beyond symptom management. They hold the key to revolutionizing both diagnosis and treatment of Parkinson’s disease, leading to earlier, more precise interventions and, ultimately, improved patient outcomes.
Challenges in Developing a Cure
Parkinson’s disease is a complex and heterogeneous condition, presenting a significant challenge for researchers and clinicians alike. With over 40 symptoms ranging from tremors and pain to hallucinations, the experience of Parkinson’s disease varies greatly from one individual to another. This variation means there may never be a single “cure” that works universally. Instead, the future of treatment lies in a combination of therapies that address the diverse needs of individuals, tailoring approaches to their specific form of the disease.
One of the major hurdles in developing a cure is the difficulty in diagnosing Parkinson’s early due to its overlapping symptoms with other neurological disorders. This often leads to delayed diagnoses, making it harder to initiate early intervention that could potentially slow disease progression. Additionally, even though current treatments help manage symptoms, they do not halt the underlying neurodegeneration and often lose effectiveness over time, requiring ongoing adjustments to the treatment plan.
Another significant challenge is the need to repair or replace damaged brain cells, as Parkinson’s causes the progressive loss of dopamine-producing neurons. While some therapies aim to alleviate symptoms, such as motor fluctuations and tremors, no current treatment addresses the root cause of the disease.
To overcome these obstacles, a multi-faceted approach is needed, involving not only pharmacological treatments but also lifestyle changes, such as diet, exercise, and complementary therapies like physical, occupational, and speech therapy. For certain patients, surgical options, including deep brain stimulation, can provide relief when medications are no longer effective.
Despite these challenges, organizations like Parkinson’s UK are accelerating the search for a cure by funding research, supporting innovative therapies, and working with global collaborators to make clinical trials more efficient. Their commitment to speeding up the development and testing of new treatments gives hope that, while a cure may not be immediate, significant advancements are on the horizon, offering better management and, eventually, a cure for Parkinson’s disease.
The road to a cure is long, but with continued investment in research and innovation, we may see breakthroughs in the years to come, offering better management and, eventually, a cure for Parkinson’s disease.
Conclusion
In conclusion, while Parkinson’s disease remains a formidable challenge with no cure currently available, the advancements in research and therapeutic development are offering a promising outlook for the future. From innovative drug delivery systems to the exploration of biomarkers for early diagnosis, the field is rapidly evolving. Emerging therapies targeting the disease’s underlying mechanisms and tailored treatments for both motor and non-motor symptoms offer hope for improved management and potentially disease-modifying options. With ongoing efforts from researchers, pharmaceutical companies, and organizations dedicated to Parkinson’s disease, the path toward more effective treatments and, ultimately, a cure is becoming increasingly tangible, promising a brighter future for patients and their families.

Downloads
Article in PDF
Recent Articles
- Most Promising Therapies in the Parkinson’s Disease Treatment Market
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- The Future of Parkinson’s Disease Treatment: Unlocking the Potential of Cell and Gene Therapy
- Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Sclet...
- AbbVie Reveals Phase III TEMPO-2 Trial Positive Topline Results; FDA Accepts GSK’s NUCALA Submiss...