PD-1 and PDL-1 Immune Check Point Inhibitors: A Prodigious Revolution in Cancer Treatment
Aug 29, 2016
Immune checkpoints are defined as the stimulatory or co-stimulatory molecules involved in the immune system. There are two kinds of checkpoint proteins found on T cells such as PD-1/PD-L1 and cytotoxic T lymphocytes (CTLA-4). The PD1/PD-L1 pathway is an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous anti-tumor activity. PD-1, a protein present in humans encoded by the PDCD1 gene, binds to two ligands- PD-L1 and PD-L2 to prevent the activation of T-cells by regulating the immune system. PD-L1 is a protein present in human encoded by CD274 gene that reduces the proliferation of CD8+ T cells at the lymph nodes by binding to PD-1 or B7.
Immune checkpoint inhibitors are type of drugs, usually antibodies, which block these proteins of the immune system cells. Inhibiting the binding of PD-L1 to PD-1 with an immune checkpoint inhibitor leads to killing of tumor cells by T Cells. PD-1 and PD-L1 inhibitors are used as immunotherapy for treating various cancers such as Lung Cancer, Metastatic Renal Cell Carcinoma, Head and Neck Cancer and Melanoma. They also protect against immune-mediated tissue damage.
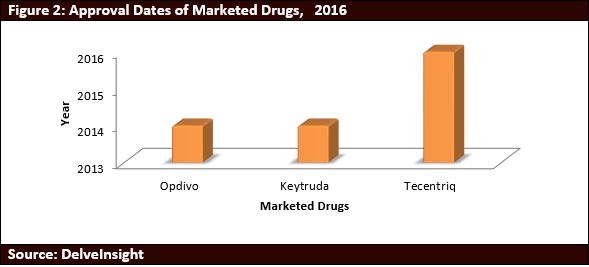
- Currently, there are 3 approved drugs for PD-1 and PDL-1 inhibitors. Tecentriz was the first PDL-1 inhibitor to be approved and other 2 drugs i.e. Opdivo and Keytruda are PD-1 inhibitors.
- Opdivo was the first PD-1 inhibitor to be approved in July 2014. It has been approved for the treatment of Advanced Melanoma, Advanced Renal Cell Carcinoma, Hodgkin lymphoma, Non-Small Cell Lung Cancer and Metastatic Keytruda has been approved for the treatment of Advanced Melanoma, Non-Small Cell Lung Cancer and Metastatic Melanoma.
- More durable effect on cancer and low toxicity is observed with Tecentriq as compared to other approved drugs.
- It is estimated that PD-1 and PDL-inhibitors bring the sales of USD 35 Billion. Opdivo had USD 2.1 billion sales versus Keytruda’s USD 566 million in 2015. It is also estimated that Tecentriq will have blockbuster sales in coming years.
PD-1 and PD-L1 inhibitors have replaced existing chemotherapy and radiotherapy for treating cancer. Several clinical trials for combination therapy of PD-1 and PD-L1 inhibitors are ongoingwith around 245 active clinical studies. The Pipeline of PD-1 and PD-L1 is profuse with approx. 47 drugs with 35 companies involved, including big pharmaceutical companies such as Hoffmann-La Roche, Merck Sharp & Dohme Corp., Pfizer Inc., Bristol-Myers Squibb and AstraZeneca.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Roche’s quantitative COVID-19 antibody test; Janssen buys a gene therapy asset; Zimmer Acqu...
- FDA approves Ofev for interstitial lung disease
- Ceribell raises $35M; Lyra reels $29.5M; Minoryx raises Euro21M; Alexion acquired Syntimmune
- AbbVie Receives Approval; Kymriah Approved; Shire Gets USFDA Approval; NICE Rejects; Bristol-Myer...
- BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra;...

Insight by:
Diksha Wadhwa
Associate Analyst
DelveInsight Buisness Research, LLP
Downloads
Article in PDF
Recent Articles
- Sanofi to Acquire Provention Bio; USFDA Committee Votes in Favor Roche’s Polivy; FDA to Rev...
- Roche’s quantitative COVID-19 antibody test; Janssen buys a gene therapy asset; Zimmer Acqu...
- Acquisition of Stemline; Roche’s Elecsys Anti-SARS-CoV-2 antibody test; Newron’s abandonment of S...
- Booming Healthcare sector in MENA: Lucrative opportunity for Global pharma players
- The Business Cocktail



