Revolutionizing Schizophrenia Treatment: The Key Players Shaping the Future
Jun 30, 2025
Table of Contents
Schizophrenia, a term introduced over a century ago, continues to perplex clinicians and researchers alike. Characterized by hallucinations, delusions, disorganized thinking, and cognitive decline, it remains one of the most debilitating and misunderstood psychiatric conditions. Affecting approximately 20 million people globally, schizophrenia poses a massive public health challenge. As per DelveInsight, the total Schizophrenia prevalent population in the 7MM was 6,029,994 in 2022, and the U.S. accounted for approximately 45.05% of the total prevalent cases, followed by EU4 and the UK with around 37.36%, and Japan with about 17.59%.
This chronic psychotic condition disrupts thought processes, impairs social functioning, and hinders cognitive development. Despite decades of research and the ongoing development of schizophrenia drugs and antipsychotic treatments, its underlying pathophysiology remains largely unclear.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- 5 Upcoming Schizophrenia Drugs Competitors for Newly Approved BMS’ KarXT (COBENFY)
- Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
- Breaking Boundaries: Innovations and Updates in Schizophrenia Treatment
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
- How are Antipsychotics Transforming the Schizophrenia Treatment Space?
Schizophrenia Market: The Present Case Scenario
At present, there is no known cure for schizophrenia; however, the condition is treatable and manageable through a combination of therapies. While treatment goals vary based on the severity and phase of illness, the immediate objective of schizophrenia treatments is to manage acute symptoms, particularly psychosis, and enhance patients’ quality of life. Over the long term, the focus shifts toward achieving clinical stability and preventing relapse.
Medications are the cornerstone of the schizophrenia drug market, with antipsychotics serving as the primary line of pharmacological treatment. The broader schizophrenia treatment market includes medications (antipsychotics), cognitive behavioral therapy (CBT), psychological counseling, social support, and electroconvulsive therapy (ECT). Antipsychotics (APs), classified as first-generation (FGA or typical) and second-generation (SGA or atypical), are commonly administered either orally (OAPs) or as long-acting injectables (LAIs). While both FGA and SGA are widely used in clinical practice, debates continue regarding the comparative effectiveness of SGAs over FGAs, as well as LAIs over OAPs.
Several FDA-approved schizophrenia medications are available, such as REXULTI (brexpiprazole), CAPLYTA (lumateperone), LATUDA (lurasidone), SAPHRIS (asenapine), ABILIFY MYCITE (aripiprazole with a digital sensor), VRAYLAR (cariprazine), SECUADO (asenapine), the INVEGA series (paliperidone), ARISTADA (aripiprazole lauroxil), PERSERIS (risperidone), FANAPT (iloperidone), LYBALVI (olanzapine and samidorphan), and others. These medications for schizophrenia continue to dominate the market, offering options tailored to various stages and symptom profiles.
COBENFY, approved in September 2024, is recognized as the first novel drug for schizophrenia in 35 years, introducing a new class of treatment by selectively targeting M1 and M4 receptors in the brain without blocking D2 receptors.
Talking about the most recent approval, in April 2025, CHA Biotech’s subsidiary CMG Pharmaceutical received FDA approval for MEZOFY (formerly Depipzo), an oral film-type treatment for schizophrenia. MEZOFY, containing aripiprazole, addresses patient non-compliance by dissolving easily in the mouth, eliminating the need for water.
Wish to Know More About the Recent Approvals of Schizophrenia Drugs? Click here.
Furthermore, antipsychotic drugs are often prescribed in combination with other drug classes such as neuroleptics and antiepileptics to manage complex symptom profiles in schizophrenia. Given the high prevalence of depression among patients, antidepressants are frequently added to the treatment regimen to address comorbid mood disorders. In some cases, research supports the use of combination therapy involving antipsychotics and benzodiazepines to help control associated symptoms like sleep disturbances, anxiety, or behavioral disinhibition. Additionally, anticholinergic drugs are commonly used alongside antipsychotics to manage extrapyramidal side effects, marking a significant component of the evolving schizophrenia treatment market.
Schizophrenia Recent Developments
- In March 2025, Vanda Pharmaceuticals Inc. announced the submission of a New Drug Application (NDA) to the FDA for marketing approval of BYSANTI (milsaperidone) for the treatment of acute bipolar I disorder and schizophrenia. The NDA is supported by multiple clinical studies evaluating BYSANTI’s efficacy and safety.
- In January 2025, Boehringer announced that its Phase III CONNEX program for the investigational schizophrenia drug iclepertin did not meet its primary or key secondary endpoints. After six months, the drug showed no significant cognitive or functional improvements compared to placebo in any of the three studies.
- In January 2025, Johnson & Johnson and Intra-Cellular Therapies announced a definitive agreement under which Johnson & Johnson will acquire all outstanding shares of Intra-Cellular Therapies, a biopharmaceutical company specializing in the development and commercialization of CNS disorder treatments, for $132.00 per share in cash, totaling approximately $14.6 billion in equity value.
- In November 2024, AbbVie announced that its two Phase II EMPOWER trials of emraclidine, a once-daily oral monotherapy for adults with schizophrenia experiencing acute psychotic symptoms, did not meet the primary endpoint. The trials showed no statistically significant improvement in the Positive and Negative Syndrome Scale (PANSS) total score compared to placebo at week 6.
- In September 2024, Bristol Myers Squibb (BMY) announced that the FDA approved COBENFY (xanomeline and trospium chloride), an oral medication for treating schizophrenia in adults. The FDA approval is especially notable as it marks the first novel drug for schizophrenia in 35 years, introducing a new class of treatment by selectively targeting M1 and M4 receptors in the brain without blocking D2 receptors. This breakthrough has the potential to reshape the treatment landscape for schizophrenia, a milestone highlighted by CEO Chris Boerner, PhD, who emphasized the significance of this novel approach in neuropsychiatry.
Bridging the Gap in the Schizophrenia Market
Decades of research have highlighted the undeniable role of schizophrenia drugs, particularly antipsychotic medications, in improving brain function and quality of life. However, these schizophrenia treatments exhibit variable effects across patients, largely due to differing affinities of these drugs toward various brain receptor binding sites. Most of these medications for schizophrenia must be continued for five years or more after stabilization to prevent relapse. Since the majority of antipsychotic drugs work through dopamine modulation, they are mainly effective in managing positive symptoms, leaving negative symptoms and cognitive impairment largely unaddressed. Additionally, they result in heterogeneous acute effects on the blood oxygen level-dependent (BOLD) signal, while the biological interpretation of these neuronal changes remains poorly understood.
Alarmingly, about 30% of patients are resistant to available schizophrenia antipsychotics, pointing to the limitations of current schizophrenia treatment drugs. Moreover, 80–90% of patients experience relapse due to nonadherence to long-term therapy. This underscores the need for intensified R&D in the schizophrenia drug market, including a better understanding of the mechanism of action (MoA) and the development of quantitative biomarkers to predict brain activity changes related to drug response. There’s a growing demand for innovative therapeutic agents that can address cognition impairment, overcome drug resistance, and effectively treat negative symptoms. In response, many schizophrenia drug companies are actively exploring novel therapies within the evolving schizophrenia pipeline to close these persistent treatment gaps.
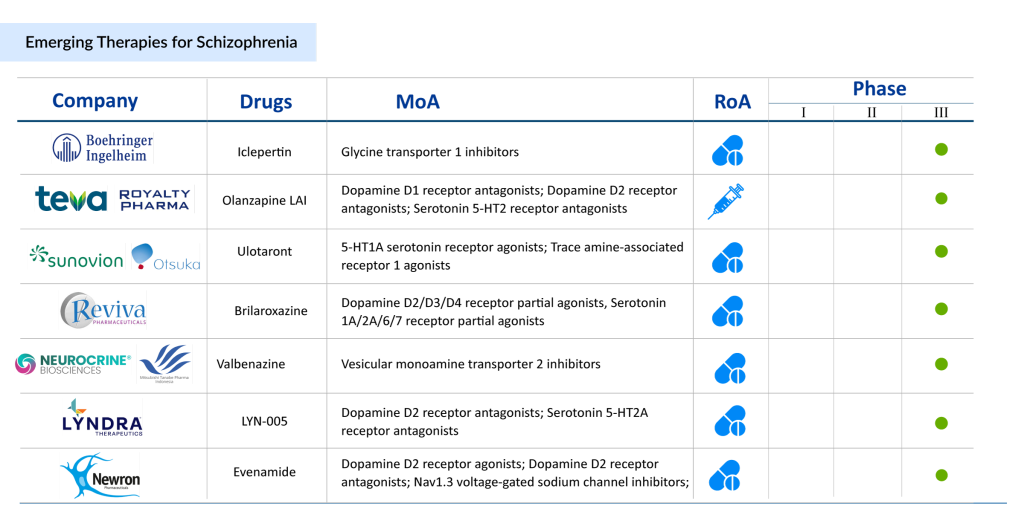
As per DelveInsight, the market size of schizophrenia in the 7MM was approximately USD 8 billion in 2023, which is expected to grow at a significant CAGR by 2034.
Various potential early-phase therapies that are projected to enter the schizophrenia market during the forecast period include AQW051 (Novartis Pharmaceuticals), JX11502MA (Zhejiang Jingxin Pharmaceutical Co., Ltd.), HS-10380 (Jiangsu Hansoh Pharmaceutical Co., Ltd., SPG302 (Spinogenix), CVL-231 (Cerevel Therapeutics, LLC), LB-102 (LB Pharmaceuticals Inc.), Luvadaxistat (Neurocrine Biosciences), and others.
Iclepertin (BI-425809) is an investigational glycine transporter 1 (GlyT1) inhibitor developed by Boehringer Ingelheim, aimed at addressing cognitive symptoms associated with schizophrenia. By enhancing NMDA receptor function, Iclepertin may improve synaptic plasticity and cognition. Currently undergoing multiple Phase III trials, it also shows potential for Alzheimer’s disease, though its earlier Phase II results for that indication were inconclusive. Ulotaront (SEP-363856), developed by Sumitomo Pharma and Otsuka Pharmaceuticals, is a trace amine-associated receptor 1 (TAAR1) agonist that uniquely does not target dopamine D2 or serotonin 5-HT2A receptors. This small-molecule oral agent is in late-stage development for schizophrenia and generalized anxiety disorder. Despite halting some Phase III trials, two are still ongoing, with a revised licensing agreement giving Otsuka exclusive rights to develop and commercialize Ulotaront worldwide. Both therapies have the potential to reshape the treatment landscape for schizophrenia, offering new hope for patients.
In a nutshell, the schizophrenia market is experiencing a transformative surge, with pharmaceutical companies accelerating efforts to develop next-generation schizophrenia drugs that promise to redefine treatment outcomes. The coming decade is set to witness a dynamic shift driven by innovative schizophrenia pipeline therapies like iclepertin, brilaroxazine, and SEP-363856, fueling significant revenue growth for leading schizophrenia drug companies. While recent strides in mental health advocacy have amplified awareness, there’s still a critical need to enhance long-term treatment success and improve the overall quality of life for those affected.
But managing schizophrenia goes beyond just prescribing medications. It’s about confronting societal misconceptions, erasing outdated stigmas, and treating mental illness with the same urgency, innovation, and empathy as any physical health condition. As schizophrenia treatments continue to evolve, combining science with compassion will be key to truly shifting the landscape. Ultimately, it’s not only the promising schizophrenia medications in the pipeline that hold the power to transform lives, but also the global mindset shift that recognizes and respects the complexity of mental health.

FAQs
In 2023, INVEGA and VRAYLAR (cariprazine) held the major market share. However, this dominance is expected to decline by 2034 due to the introduction of new therapies.
Various potential early-phase therapies that are projected to enter the schizophrenia market during the forecast period include AQW051 (Novartis Pharmaceuticals), JX11502MA (Zhejiang Jingxin Pharmaceutical Co., Ltd.), HS-10380 (Jiangsu Hansoh Pharmaceutical Co., Ltd., SPG302 (Spinogenix), CVL-231 (Cerevel Therapeutics, LLC), LB-102 (LB Pharmaceuticals Inc.), Luvadaxistat (Neurocrine Biosciences), and others.
The schizophrenia market across the 7MM (United States, EU4, the UK, Japan) was valued at approximately USD 8 billion in 2023 and is projected to grow significantly by 2034.
Downloads
Article in PDF
Recent Articles
- US/AZ deal; Alkermes’ Schizophrenia drug; Innovent/Lilly’s China market expansion; Re...
- How are Antipsychotics Transforming the Schizophrenia Treatment Space?
- 5 Upcoming Schizophrenia Drugs Competitors for Newly Approved BMS’ KarXT (COBENFY)
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
- Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval