CRISPR/Cas9 gene
Aug 22, 2025
Vertex & CRISPR Therapeutics Make History: FDA Approves exa-cel, the First CRISPR Gene Therapy for Sickle Cell and Beta-Thalassemia
Vertex Pharmaceuticals and CRISPR Therapeutics have become the first companies to secure FDA approval for a CRISPR-based gene-editing therapy. Vertex Pharmaceuticals, in partnership with CRISPR Therapeutics, has achieved a historic milestone with the FDA approval of exagamglogene autotemcel (exa-cel, branded as ...
Read More...
Dec 15, 2023
Lyfgenia or Casgevy: Who Will Lead the Sickle Cell Disease Treatment Space?
History has been created as the world captures the significance of the FDA’s approval of Casgevy (exa-cel), a collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, for the treatment of sickle cell disease (SCD). This groundbreaking therapy represents a long-awaited potential cure for the debilitatin...
Read More...
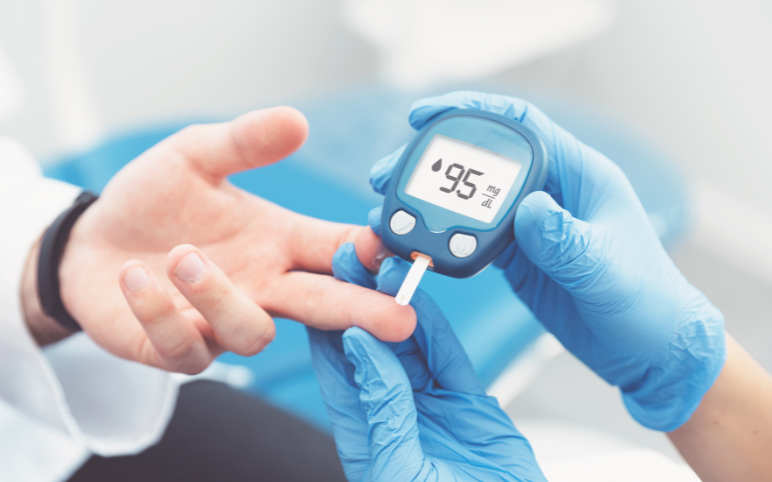
May 01, 2023
Cell and Gene Therapies for Diabetes Treatment: A Permanent Cure for Patients?
Diabetes is the 8th largest cause of death in the United States (although its prevalence may be underreported). Diabetes affects more than 37 million people in the United States, and 1 in every 5 are unaware of their condition. Over 96 million US adults—more than one-third—have prediabetes, and more than 8 out of 1...
Read More...
Jun 10, 2022
Unraveling the Potential of CRISPR Technology in the Gene-editing Space
CRISPR technology or CRISPR/Cas9 technology is a genome-editing tool derived from the bacterial defense system against viruses and plasmids. The system comprises the Cas9 nuclease enzyme to create site-directed dsDNA (double-stranded DNA) break and a guide RNA, which is a predesigned 20 bp long RNA sequence within ...
Read More...
Feb 14, 2018
Business Cocktail
Roivant expanding its horizon into metabolic diseases segment with USD 650 million diabetes pact with Poxel Roivant Sciences has recently signed a USD 650 million deal to acquire near-global rights to Poxel pharma’s phase 3-ready Type 2 diabetes drug (imeglimin). It’s been a while since Poxel was waiting f...
Read More...




-Agonist.png)

