All Blogs
Jul 14, 2021
Key Pharma Companies Expanding their Arm in the Skin Cancer Segment
Cancer today is one of the most deadly diseases worldwide, causing a significant burden in terms of mortality, years of life lost, and disability as compared to other diseases. It hampers economic development, weighs down the healthcare system of the country, and affects the quality of life of the patient and their...
Read More...
Jul 13, 2021
Bayer’s CKD Drug; Enochian’s RNA Hijack for HBV; Novo Nordisk’s to get Prothena’s ATTR Amyloidosis Business; J&J’s COVID-19 Vaccine Struggle
Bayer Scores FDA-Approval for its CKD Drug Finerenone Following a priority review, the USFDA finally gave its thumbs-up to Bayer AG’s Kerendia (finerenone) for chronic kidney disease (CKD) associated with type 2 diabetes (T2D) based on the results from the Phase III FIDELIO-DKD study. Finerenone is the first ...
Read More...
Jul 12, 2021
With advancements in Innovative Technology, Cardiac Monitoring Devices Market is Booming Significantly
Cardiac monitoring devices are important for cardiovascular care, to assess the occurrence and seriousness of cardiac failure and to evaluate the success of therapies such as medications, procedures, and device implants. Cardiovascular diseases (CVDs) are a global cause of death, which can be prevented. In most cas...
Read More...
Jul 09, 2021
JAK Inhibitors Market
JAK inhibitors also termed “Jakinibs,” work by inhibiting the activity and response of one or more Janus kinase enzymes. These inhibitors function against the Janus kinases, a group of four proteins that includes JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). These proteins control inflammation by triggering intra...
Read More...
Jul 08, 2021
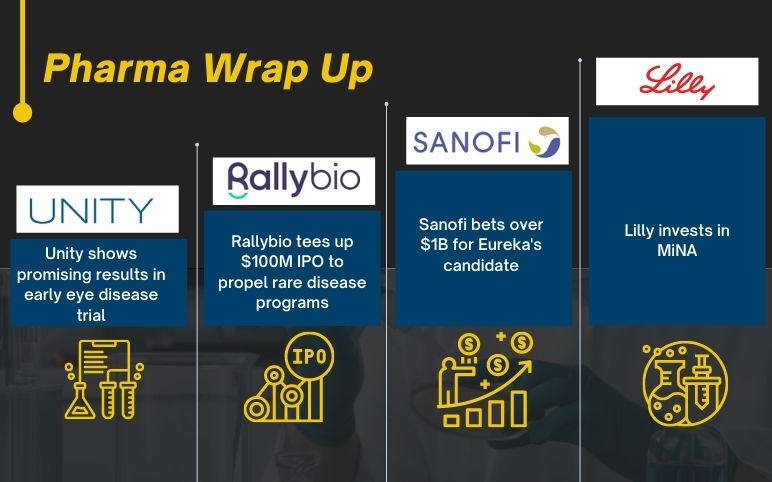
Unity’s advanced vascular eye disease trial; Rallybio raises $100 M; Sanofi bets over $1B for Eureka’s candidate; Lilly invests in MiNA;
UNITY Biotechnology declares positive data from phase 1 clinical trial of UBX1325 in Advanced Vascular Eye Disease patients UNITY Biotechnology, Inc., a biotechnology company developing therapeutics to decrease, impede, or reverse diseases of aging, declared positive data from its Phase 1 safety study of U...
Read More...
Jul 07, 2021
Charting out the COVID-19 Vaccine Landscape
The uncontrolled outbreaks of Covid-19 have brought unprecedented challenges to the world community. It has created an imbalance in mental, social, and economic well-being. Apart from this, it has changed the overall way of living and has created fear and anxiety among people. Countries are experiencing peaks, one ...
Read More...
Jul 06, 2021
Arrowhead’s ARO-ENaC Clinical Trial; Sartorius Acquires Stake in CellGenix; AGC Biologics’s Cell & Gene Therapy; Sirnaomics Secures $105 Million
Arrowhead Halts its ARO-ENaC Phase 1/2 Clinical Trial Arrowhead Pharmaceuticals recently notified about its voluntary decision to put a pause on its ongoing Phase 1/2 clinical study of ARO-ENaC for patients with cystic fibrosis (CF). The announcement came after a preliminary update from an ongoing chronic ...
Read More...
Jul 05, 2021
Neurovascular Thrombectomy Devices Market
Neurovascular thrombectomy devices are used for eliminating the blood clots that have accumulated in the neurovascular vessels during an acute ischemic stroke. These devices comprise a wide range of endovascular tools that are capable of removing thrombi from Neurovasculature in the case of patients suffering from ...
Read More...
Jul 02, 2021
Vagus Nerve Stimulators Market Outlook
Vagus nerve stimulators (VNS) is a neuromodulation therapy approved by the United States Food and Drug Administration (FDA) as a palliative treatment for patients with both epilepsy and depressions. Other non-FDA-approved uses of VNS have been studied and explored in various small open-labeled studies which include...
Read More...
Jul 01, 2021
MedTech Market Industry: Approval of Devices, Therapy Address Market Needs | Clinical Outcomes Create New Value
LivaNova Secures FDA Approval for Clinical Study to Assess the aura6000 System for Obstructive Sleep Apnea Treatment On June 15, 2021, LivaNova PLC received approval from the US Food and Drug Administration (FDA) to continue with its investigational device exemption (IDE) clinical study, “Treating Ob...
Read More...