Off-label Therapies Dominating the Behcet’s Syndrome Treatment Market
Jan 13, 2023
Table of Contents
Behcet’s syndrome is an uncommon multisystem inflammatory ailment marked by ulcers in the mouth and genitalia, different skin lesions, and eye abnormalities. It is linked to several new clinical symptoms, all caused by an underlying vasculitis that affects arteries of all diameters.
The HLA-B*5101 allele of HLA-B51 and other non-HLA genes are linked to Behcet’s syndrome. Patients from these regions have more comparable clinical characteristics than patients from the west, whose condition has a weaker connection with HLA antigens. Although familial Behcet’s syndrome is rare and has a clear pattern of inheritance, 15% of affected children have the familial condition.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Merck’s Remicade; Sanofi, Regeneron set Kevzara; Novartis to sever; Teva puts women’s...
- Analyzing the Growth of the Biosimilar Market Through Years
- Idera’s Phase I Data; DelMar Initiates Trial; Novartis’ study; Humira gets EC approval
- HUMIRA Biosimilars in the US: The Talk of the Psoriatic Arthritis Treatment Market
- Gilead faces lawsuit; Herceptin stalled; Lilly revs up, Abemaciclib gets nod; Key Humira patent g...
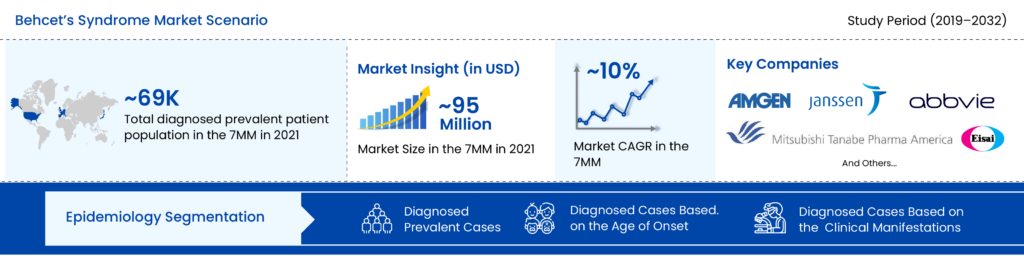
As per the DelveInsight assessment, in 2021, the total diagnosed prevalent cases of Behcet’s syndrome was more than 69K in the 7MM. The highest number of cases was seen in Japan.
The apex of the Behcet’s syndrome treatment landscape
The Behcet’s syndrome treatment market was valued at around USD 94 million in 2019 and is expected to reach USD 270 million by 2032, growing at a CAGR of ~10% during the study period.
Owing to the rarity of the disease, the choice of Behcet’s syndrome treatment depends on the physician. Currently, off-label therapies sit at the top of Behcet’s syndrome treatment landscape. Agents like colchicine, corticosteroids, azathioprine, anakinra, canakinumab, secukinumab, and ustekinumab are looked upon to manage Behcet’s syndrome.
Corticosteroids are used to treat inflammation, azathioprine is recommended in patients with recurrent arthritis, and glucocorticoids are considered during acute exacerbations. OTEZLA is the first US FDA-approved Behcet’s syndrome therapy to treat oral ulcers associated with Behcet’s syndrome. REMICADE (infliximab) and HUMIRA (adalimumab) are incorporated to treat refractory uveoretinitis in Behcet’s and intestinal Behcet’s disease.
The current Behcet’s syndrome treatment landscape presents an unmet need for approved therapies for treating Behcet’s syndrome.
Low diagnosis, lower patients, lowest development in Behcet’s syndrome treatment landscape
Early Behcet’s syndrome diagnosis continues to be extremely difficult without a reliable biological marker and an exact diagnostic test. Due to the insufficient scientific evidence and few cases reported leading to no prospective Behcet’s syndrome clinical trials, it presents a hurdle in developing new treatment modalities. Access to medicines that can effectively treat specific illness presentations remains a key barrier due to the lack of sizable randomized controlled clinical studies to examine the impact of various interventions in Behcet’s syndrome.
Behcet’s syndrome treatment procedures for the vascular, neurologic, and gastrointestinal association are mostly founded on uncontrolled investigations. In this manner, future RCTs and multicenter observational examinations in these fields must further develop proof levels for treatment proposals in these areas.
Key developments in the Behcet’s syndrome treatment space so far
- In February 2020, the EMA granted MAA to OTEZLA to treat adult patients with oral ulcers associated with Behcet’s syndrome who are candidates for systemic therapies.
- In September 2019, the PMDA granted approval for OTEZLA to treat oral ulcers associated with Behcet’s syndrome in patients who have not responded sufficiently to local treatment.
- In July 2019, Amgen announced that the US FDA approved OTEZLA (apremilast) 30 mg BID for treating adult patients with oral ulcers associated with Behcet’s syndrome.
Expected roadblocks in the Behcet’s syndrome treatment market
The lack of specific diagnostic approaches, such as laboratory tests, biomarkers, and updated diagnostic criteria that account for the neurological, GI, and vascular complications associated with Behcet’s syndrome, leaves clinicians in a bind. Moreover, there are no specific consensus-driven recommendations for all manifestations of Behcet’s syndrome that take gender or age into account, as age and early disease can influence the treatment of complications associated with Behcet’s syndrome.
Although the discovery of TNF inhibitors’ role in Behcet’s syndrome opened up new opportunities in Behcet’s syndrome treatment market, the reluctance of patients and physicians to begin new biologics due to safety concerns is a major setback for biologics. According to research, even though approximately 60% of patients achieve remission after the first few years, Behcet’s syndrome is associated with high relapse rates, a poor prognosis, and mortality.
Another impediment to researching potential new and existing therapies is a lack of understanding of Behcet’s syndrome pathogenesis. While there is a large body of literature on the possible mechanisms of Behcet’s syndrome, understanding whether these are influenced by environmental, genetic, or other factors is still very limited. Due to the disease’s complexity and multisystem involvement, a holistic approach to management with the involvement of specialists is required.
The optimistic future of the Behcet’s syndrome treatment market
As Behcet’s syndrome demands more advancements to bridge the gap between research and Behcet’s syndrome treatment, more interventional studies are required to understand the disease and establish treatment options. According to research articles, Behcet’s syndrome is widespread along the old Silk Road, which extends from eastern Asia to the Mediterranean, but because of the migration of Turks to Germany and other European nations, it might encourage research into the condition.

FAQs
Behcet’s syndrome is an uncommon multisystem inflammatory ailment marked by ulcers in the mouth and genitalia, different skin lesions, and eye abnormalities. It is linked to several new clinical symptoms, all caused by an underlying vasculitis that affects arteries of all diameters.
The Behcet’s syndrome symptoms include mucous membrane lesions of the mouth (canker sores) and genitals (ulcers), which disappear and reappear on their own. Individuals with Behcet’s syndrome may also experience eye inflammation (anterior uveitis, posterior uveitis, or panuveitis).
There are no specific tests or biomarkers that can confirm a Behcet’s syndrome diagnosis. Clinical criteria, the occurrence of disease signs and symptoms, and positive clinical criteria are referred to as the International Study Group (ISG) criteria for Behcet’s disease.
The only therapy approved by US FDA is OTEZLA (apremilast) for treating oral ulcers associated with Behcet’s syndrome. In Japan, REMICADE (infliximab) and HUMIRA (adalimumab) are approved for refractory uveoretinitis in Behcet’s disease and intestinal Behcet’s disease; however, several off-label therapies, such as colchicine, corticosteroids, azathioprine, anakinra, canakinumab, secukinumab, and ustekinumab, are used for the management of Behcet’s syndrome.
Downloads
Article in PDF
Recent Articles
- Humira’s patent; Teva laying off; GSK aims; Thermo acquires Patheon; J& J’s Invokana
- 7 Hidradenitis Suppurativa Drugs Set to Hit the Market in the Next 5 Years
- Merck to Acquire Harpoon Therapeutics; Novo Nordisk Enters Into Collaborations with Omega Therape...
- Merck’s Remicade; Sanofi, Regeneron set Kevzara; Novartis to sever; Teva puts women’s...
- How Novel Therapies Could Transform the Ulcerative Colitis Treatment Landscape