Cancer Biomarkers: Improving Treatment and Detection
Mar 13, 2023
Table of Contents
Cancer is the second leading cause of death worldwide, after ischemic heart disease. There are approximately 200 different types of cancer, with lung, prostate, colorectal, stomach, and liver cancer being the most common in men and breast, colorectal, lung, cervical, and thyroid cancer being the most common in women. Every year, approximately 10 million people die due to cancer. More than 40% of cancer-related deaths may be avoidable due to modifiable risk factors such as smoking, alcohol use, poor diet, and physical inactivity. Around 70% of cancer deaths occur in low-to-middle-income countries. Millions of lives could be saved annually if resource-appropriate prevention, early detection, and treatment strategies were implemented. Moreover, the total annual economic cost of cancer treatment is estimated to be $1.16 trillion. In addition, there is a significant disparity in treatment availability and outcome across countries and socioeconomic groups, resulting in a higher cancer burden on patients and their families.
Rising Cancer Patient Burden
The global cancer burden is increasing, putting enormous physical, emotional, and financial strain on individuals, families, communities, and healthcare systems. Many health systems in low- and middle-income countries are unprepared to handle this burden, and a large number of cancer patients worldwide do not have timely access to quality diagnosis and treatment. Survival rates for many types of cancer are improving in countries with robust health systems, thanks to more accessible early detection, quality treatment, and survivorship care.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Apollomics raises; Pear Therapeutics raises $64M; Lilly to acquire Loxo
- Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant fo...
- Are you ready to embrace Gene Therapy?
- AACR 2022: LAG-3, one of the most trending Next-generation Immunotherapy
- The promising Pipeline for Gene Therapy In Oncology
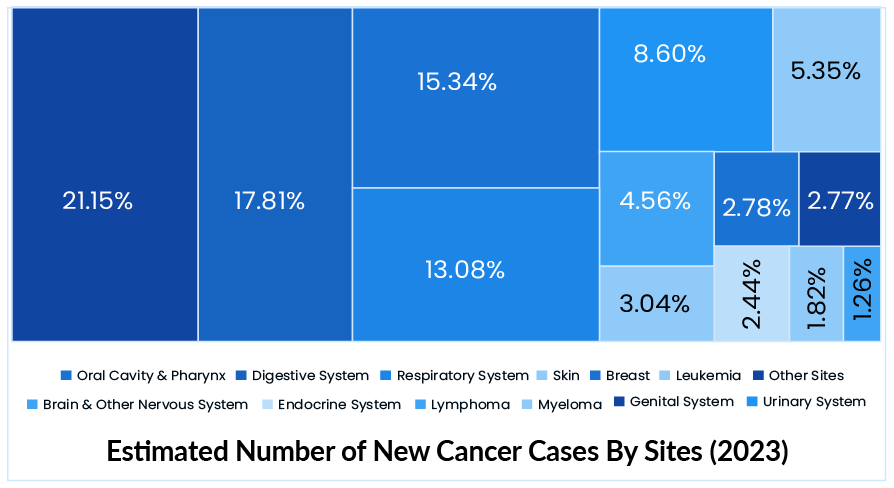
In 2022, there were an estimated 1.9 million new cancer cases and 609K cancer deaths in the US. Female breast, lung, colorectal, prostate, and stomach cancers account for roughly 50% of all cases. Moreover, in 2023, there will be an estimated 2 million new cancer cases and 610K cancer deaths in the US. Lung cancer is by far the most common cause of cancer death, accounting for 1 in every 5 deaths. The global cancer burden is expected to reach 28.0 million new cases and 16.2 million cancer deaths by 2040, owing solely to population growth and aging.
Cancer Management and Therapy: Current Trends and Challenges
Curing cancer is undeniably one of the century’s major challenges. Cancer treatment can never be “one size fits all” because there are so many different types of cancers and their behaviors. Several factors influence treatment choice, including the type of cancer, the site or organ of origin, the cancer stage, the patient’s age and overall health at the time of diagnosis, tumor pathology, and immunohistochemistry. NSCLC, for example, is currently treated with surgery, radiation, immunotherapy, or chemotherapy.
Aside from the basic criteria, cancer, and its treatment approaches differ significantly from country to country; therefore, oncology marketers must understand not only the biology of each disease but also the global differences. This could be due to differences in technological advancement, the rate of research and development, or various other factors. The primary goal of cancer treatment is to cure cancer and restore the ability to live a normal life. Depending on the circumstances, the treatments may be used to reduce or slow cancer progression, allowing patients to live as long as possible without symptoms. The main treatment options used to achieve the primary goal of cancer treatment, which is eradicating all cancer cells from the body or completely removing cancer from the body, are radiation therapy or chemotherapy. In addition, many adjunct therapies, such as hormone therapy, can be used in conjunction with the primary treatment. Palliative care is primarily used in cancer to alleviate the side effects of treatment or signs and symptoms caused by cancer.
There is currently no cure for cancer. However, recent advances in medication and technology have paved the way for newer cancer treatments, bringing us closer to a cure. As a result, there are few options for cancer treatment, and for the past half-century, non-surgical cancer treatment has been driven by two main types of traditional therapies, including chemotherapy and radiation therapy, which can be used alone or in combination. Chemotherapy is the use of medications that can kill cancer cells. While radiation therapy is the process of killing cancer cells and shrinking tumors by using high-energy radiation such as x-rays, gamma rays, neutrons, protons, and other sources.
Chemotherapies are classified according to their mechanism of action. However, recent advances in cancer pathogenesis research and development have identified numerous pathways which have been an important parameters in shaping cancer treatment. Conversely, immunotherapy has become a focal point for cancer treatment by improving the prognosis of many patients with a wide range of hematological and solid malignancies. Immunotherapy has transformed the way cancer patients are treated. It is a biological medicine made of living cells that use the power of the body’s immune system to recognize, attack, and eliminate cancer. There are various types of immunotherapy, such as vaccines, checkpoint inhibitors, and CAR T-cell therapy.
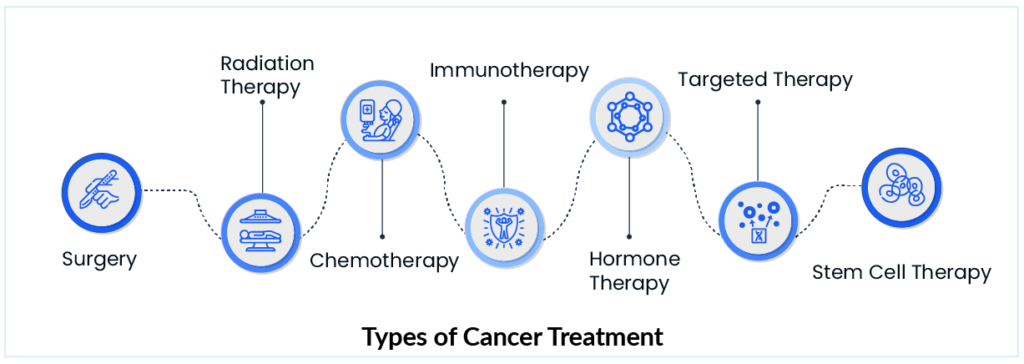
Aside from the widely used treatment methods, many other approaches, such as surgery, hormone therapy, and stem cell therapy, are included in the cancer treatment paradigm. Many treatment approaches have emerged due to years of research, but there are well-known roadblocks in the way of these treatments’ success.
Cancer Biomarkers: A New Milestone in Cancer Research
Despite advances in cancer treatment, some people do not respond to traditional chemotherapy treatment paradigms or experience disease recurrence. Biomarkers are becoming more important in the clinical care of cancer patients as genomic profiling technologies and selective molecular targeted therapies advance. The rate of development has been unusually rapid in recent decades due to a combination of factors, including an increased emphasis on precision drug development based on biomarker segmentation. Cancer treatment will continue to be redefined by new treatment therapies and techniques; for example, the discovery of biomarkers is one such landscape. The use of biomarkers in cancer treatment has changed the course of treatment dramatically.
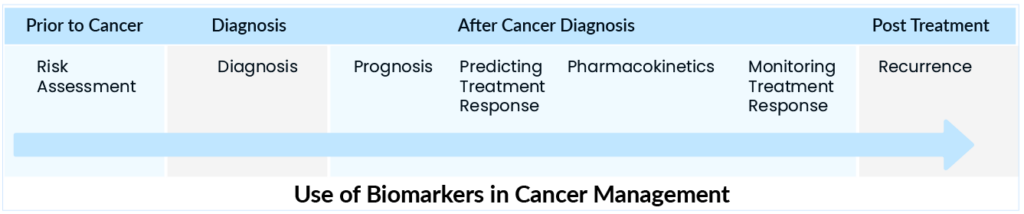
Cancer biomarkers have the potential to know the present and predict the prognosis of cancer’s future post-treatment. Cancer biomarkers can be used to assess and manage the disease at different stages accurately. They can predict various outcomes during an illness, such as early detection, outcome prediction, and disease recurrence detection. Specifically, with the introduction of numerous novel therapeutic drugs in clinical trials, appropriate markers can be used to predict which cancers will respond to specific treatments and anticipate drug resistance.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biomarkers can be useful in determining the best cancer treatment option and predicting response to therapy when combined with existing cancer treatments. Trastuzumab (Herceptin®), for example, is a targeted therapy that targets the protein HER2. Identifying and blocking the specific protein can thus aid in breast cancer treatment. Recognizing their potential involvement could also be extremely beneficial in improving cancer patient care. The use of biomarkers to assist physicians at every stage of disease management is likely to be crucial in the future of cancer management. The oncology treatment landscape is distinguished by higher success rates in rare cancers, where biomarkers can better guide therapy.
Emerging Cancer Biomarker-Specific Therapies in the Market
In recent years, research and development have anticipated incorporating cancer biomarkers to design biomarker-based therapies for cancer treatment to provide moderate benefits to patients. However, far fewer therapies have been approved for commercialization. AstraZeneca and Merck, for example, received FDA approval for lynparza (olaparib) as an adjuvant treatment for germline BRCA-mutated (gBRCAm) patients with HER2-negative high-risk early breast cancer who have already received chemotherapy before or after surgery. It is the first and only drug specifically targeting BRCA mutations in early breast cancer.
In the case of breast cancer only, triple-negative breast cancer is associated with the worst prognosis. Chemotherapy resistance is a significant problem in cancer, and many molecular mechanisms are involved in this chemo-resistance. The identification of such mechanisms is clinically important to improve prognosis. As a result, identifying cancer biomarkers predicting resistance to specific chemotherapeutic drugs is critical. These predictors can help guide treatment for both early and late-stage diseases. Many cancer biomarkers have recently been identified in the case of certain cancers, such as breast and NSCLC. Many late-stage therapies are also on the cancer biomarkers market to change the disease scenario. However, in the case of other cancers, the discovery of cancer biomarker-based therapies is still lacking, and much more research and development are required.
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||
Challenges in the Discovery of Cancer Biomarkers
There are biological and economic constraints to developing cancer biomarkers for screening, early detection, and therapy monitoring. The nature of cancer as a heterogeneous disease presents a challenge for cancer biomarkers development, and this heterogeneity may impede biomarker development. Tumors can also have multiple cancer clones with distinct molecular changes that respond differently to targeted therapy. One of the first predictive indicators for treatment selection was the BCR-ABL fusion gene. In the 1960s, a translocation between chromosomes 9 and 22 (the Philadelphia chromosome) was discovered in CML patients. It was later shown to predict response to imatinib, which the FDA approved for this indication in 2001. It took 41 years of research to find the right drug for the biomarker.
Furthermore, once the analytical validity has been confirmed, the biomarker assay in the clinical deployment platform must be examined to confirm its efficacy in predicting or diagnosing the clinical phenotype or outcome of interest, as demonstrated during the discovery and initial validation phases. However, due to cost constraints and limited patient group availability, it is realistically impossible to examine all prospective biomarkers in this manner.
Other challenges include the costs of implementing and validating newer tools in diagnostic laboratories, the bioinformatics efforts required to integrate and validate data, and, finally, the regulatory burden associated with accelerating the implementation of new technologies as a variety of validated molecular profiling platforms enter routine clinical practice. The biomarker challenge is to discover unique chemical fingerprints in complex biological mixtures that can be reliably linked to biological events to validate novel drug targets and predict treatment responses.
Future Perspective of the Cancer Biomarkers
The oncology treatment paradigm is rapidly changing with the increasing use of genetic profiling to identify essential targets involved in cancer’s hallmarks. To avoid treating patients with ineffective and toxic drugs, the development of novel therapies, particularly those targeting important molecular pathways, will necessitate systematic and reasonable planning. Cancer biomarkers have changed the way many cancer types are treated. They hold a lot of promise for patients, particularly in terms of personalized care, and better biomarkers should lead to better outcomes and more efficient, safe, cost-effective, and evidence-based utilization of health resources in the long run.
The shortcomings of existing cancer biomarkers have been known for many years. Even as new cancer biomarkers emerge, tested assays remain the gold standard for cancer screening, prognosis, and monitoring. Increasing the reliability of existing assays by combining established biomarkers with new, complementary biomarkers may be the best solution.
Advances in research techniques such as next-generation sequencing, single-cell RNA sequencing, and artificial intelligence should allow for a better understanding of the various components of the tumor microenvironment and their interactions, and potential biomarkers could be screened on a genomic scale to identify treatment efficacy predictors. As critical cancer drivers, cancer treatment can expect the development of many more targeted therapies with the help of cancer biomarkers in the coming years.
As per DelveInsight analysis, the global cancer biomarkers market is expected to grow at a CAGR of 13.73% from 2022 to 2027. The cancer biomarkers market is experiencing positive market growth as a result of an increase in the prevalence of various types of cancers worldwide, a growing population with risk factors for cancer development such as smoking and a high-fat diet, among others, and increased research and development activities in the cancer biomarkers arena leading to new product launches and increasing regulatory approvals, which are expected to result in appreciable revenue growth in the coming years.

Downloads
Article in PDF
Recent Articles
- “Endometrial Cancer – Pipeline Insights, 2015”- A DelveInsight’s Report
- ASCO GU 2022: Renal Cell Carcinoma Highlights
- Why should every company working on CAR-T therapy attend CAR TCR Summit Europe?
- AstraZeneca and Incyte Collaborate; Tocagen’s therapy; FDA warns companies;FDA Revokes
- PD-1 and PD-L1 Inhibitors: Will They Dominate Over Conventional Cancer Therapies?




