ENHERTU Plus Pertuzumab Approved in Breast Cancer: First New First-Line Option in Over a Decade
Dec 26, 2025
The pharmaceutical landscape for HER2-positive metastatic breast cancer has undergone a significant transformation with the FDA’s approval of ENHERTU (fam-trastuzumab deruxtecan-nxki) in combination with pertuzumab as a first-line treatment—marking the first new therapeutic option approved in more than a decade for this indication. This landmark approval represents a major clinical victory for patients with this aggressive form of breast cancer and reflects years of rigorous clinical research and scientific innovation.
HER2-positive metastatic breast cancer remains one of the most challenging oncological conditions to manage. Approximately one in five breast cancer cases are classified as HER2-positive, representing an aggressive disease driven by overexpression or amplification of the HER2 protein. Despite the existence of targeted therapies, the prognosis has remained sobering, with most patients experiencing disease progression within two years after receiving the current standard first-line regimen of taxane, trastuzumab (HERCEPTIN), and pertuzumab (PERJETA)—commonly referred to as THP therapy.
For years, healthcare providers lacked a more effective first-line option to address this unmet clinical need. The approval of ENHERTU Plus pertuzumab directly addresses this gap, offering patients a substantially improved treatment pathway.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMP...
- Ipsen to Buy Epizyme; BioMarin’s Gene Therapy for Hemophilia; AbbVie’s Qulipta for Ch...
- Notizia
- FDA Approves AstraZeneca’s Enhertu; Bayer Wins FDA Approval for Prostate Cancer Therapy, Nubeqa; ...
- While AZ resumes its COVID-19 vaccine trial in the UK; Merck and Gilead are busy making sizeable ...
ENHERTU represents a sophisticated advancement in cancer therapeutics. The ENHERTU drug is a HER2-directed antibody-drug conjugate engineered using Daiichi Sankyo’s proprietary DXd ADC technology platform. This innovative design consists of a HER2-targeting monoclonal antibody attached to multiple topoisomerase I inhibitor payloads via tetrapeptide-based cleavable linkers.
The mechanism of action is elegantly designed: the HER2 antibody component ensures precise targeting of cancer cells expressing the HER2 protein, while the topoisomerase I inhibitor payloads deliver cytotoxic effects by damaging cancer cells’ genomes, ultimately killing the malignant cells. This dual-action approach maximizes therapeutic efficacy while potentially minimizing systemic toxicity.
Since its initial FDA approval in December 2019 for third-line HER2-positive breast cancer, ENHERTU has continued to accumulate regulatory wins and clinical indications, establishing itself as a transformative therapy across multiple tumor types and treatment settings.
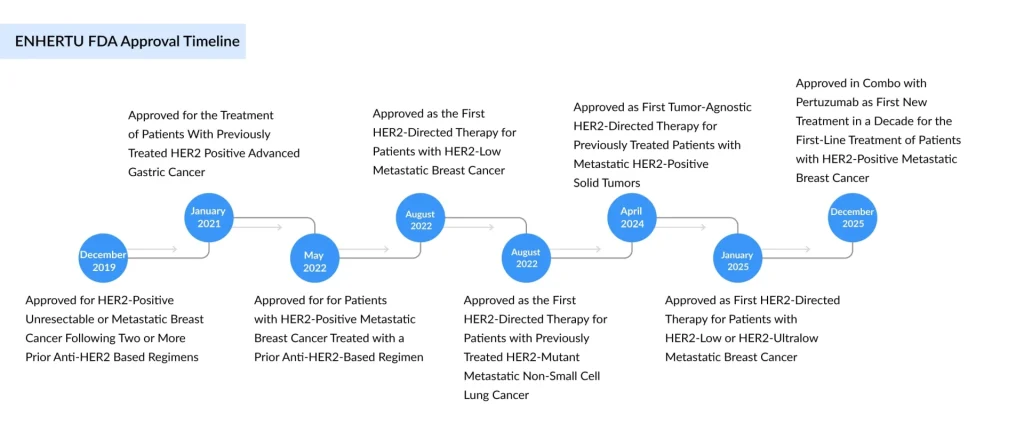
The FDA’s approval was built upon compelling data from the DESTINY-Breast09 phase 3 trial, a global, multicenter, randomized study that enrolled 1,157 patients across multiple continents. The trial compared ENHERTU in combination with pertuzumab against the standard THP regimen in patients with HER2-positive unresectable or metastatic breast cancer who had not received prior chemotherapy or HER2-targeted therapy—or had received it more than six months prior.
The clinical benefits demonstrated in DESTINY-Breast09 were nothing short of transformative. ENHERTU combined with pertuzumab reduced the risk of disease progression or death by 44% compared to THP therapy (hazard ratio: 0.56; 95% confidence interval: 0.44-0.71; p<0.0001). This translates to substantially improved survival outcomes for patients.
Median progression-free survival (PFS) emerged as a key metric of the treatment’s superiority. Patients receiving ENHERTU plus pertuzumab achieved a median PFS of 40.7 months, more than three years, compared to just 26.9 months for the THP control arm. This represents a median improvement of approximately 13.8 months, a clinically meaningful extension of the time patients remain free from disease progression.
The trial also demonstrated strong objective response rates (ORR). The ENHERTU plus pertuzumab combination achieved an ORR of 87% (95% CI: 83-90%) compared to 81% (95% CI: 77-85%) with THP. More impressively, complete responses were observed in 15% of patients receiving the combination therapy, compared with 8% in the control arm, and 72% achieved partial responses in the treatment group, compared with 73% in the control group. The median duration of response exceeded 39 months, providing patients with extended periods of symptom control and disease stability.
Dr. Sara Tolaney, Chief of the Division of Breast Oncology at Dana-Farber Cancer Institute and principal investigator for DESTINY-Breast09, captured the significance of these findings: “Trastuzumab deruxtecan plus pertuzumab is the only first-line treatment approved in more than a decade to demonstrate a statistically significant improvement in progression-free survival over the current standard regimen for patients with HER2-positive metastatic breast cancer. With a median progression-free survival exceeding three years versus approximately two years with THP, trastuzumab deruxtecan combined with pertuzumab should become a new first-line standard of care in this setting.”
ENHERTU’s path to this first-line approval reflected the FDA’s recognition of its clinical importance. The application received Priority Review, signifying the FDA’s determination that the drug offered a significant improvement over existing breast cancer therapies. Additionally, the application received Breakthrough Therapy Designation, a designation reserved for drugs demonstrating preliminary clinical evidence of substantial improvement over existing therapies for serious or life-threatening conditions.
The FDA completed its review under its Real-Time Oncology Review (RTOR) program, an initiative designed to ensure safe and effective treatments reach patients as early as possible by allowing concurrent review of data as the company submits it rather than requiring a complete submission before review begins. This expedited pathway reflected confidence in the drug’s safety and efficacy profile.
The submission was also reviewed under Project Orbis, a framework allowing concurrent submission and review of oncology medicines among participating international regulatory partners, facilitating global access to this important therapeutic option.
This approval has profound implications for clinical practice. Dr. Ken Keller, Global Head of Oncology Business and President and CEO of Daiichi Sankyo, noted: “Since its initial approval six years ago, ENHERTU has transformed the treatment of HER2-positive metastatic breast cancer. With this approval in the first-line metastatic setting, ENHERTU once again offers significant improvements in progression-free survival and has practice-changing potential when used in combination with pertuzumab.”
Dave Fredrickson, Executive Vice President of the Oncology Hematology Business Unit at AstraZeneca, emphasized the significance: “With this approval, we are bringing ENHERTU to the earliest setting for HER2-positive metastatic breast cancer, where optimizing efficacy has an important impact on long-term outcomes. The treatment approach with ENHERTU plus pertuzumab in DESTINY-Breast09 sets a new benchmark of more than three years without disease progression or death for patients in this setting.”
The approval is expected to reshape treatment algorithms for newly diagnosed HER2-positive metastatic breast cancer. Physicians will likely transition many patients from the THP regimen to ENHERTU plus pertuzumab as the new first-line standard of care, particularly for patients who can tolerate the associated toxicity profile and who do not have significant contraindications.
In a nutshell, the FDA approval of ENHERTU plus pertuzumab as a first-line treatment for HER2-positive metastatic breast cancer represents a significant clinical milestone, ending a 12-year gap since the previous first-line therapy approval. With median progression-free survival exceeding three years and a 44% reduction in disease progression or death risk compared to standard therapy, this combination offers patients meaningful improvements in survival outcomes and extended periods of disease control.
While the toxicity profile requires careful patient selection, close monitoring, and proactive management, the substantial clinical benefits position ENHERTU plus pertuzumab as a practice-changing therapy poised to become the new first-line standard of care for HER2-positive metastatic breast cancer. This ENHERTU approval underscores the continued innovation in oncology therapeutics and the potential of sophisticated antibody-drug conjugate platforms to transform cancer treatment outcomes.

Downloads
Article in PDF
Recent Articles
- CDK 4/6 Inhibitors- The Changing Paradigm for Breast Cancer Treatment
- Roche’s HER2-Positive Breast Cancer Treatment Franchise
- Metastatic HER2-Positive Breast Cancer Infographic
- How HR+/ HER2-Breast Cancer Emerging Drugs Will Transform The Market?
- Anbogen’s ABT-301 Cleared by FDA for Phase I/II Colorectal Cancer Trial; Dyne’s DYNE-251 Gets FDA...