Seagen’s TUKYSA Powers Pfizer’s Next HER2-Positive Breast Cancer Milestone
Oct 17, 2025
Pfizer’s oncology pipeline has achieved a second positive phase III trial in HER2-positive breast cancer within roughly a year. This latest success involves TUKYSA, a HER2-targeted tyrosine kinase inhibitor that Pfizer acquired through its $43 billion purchase of Seagen.
In patients with HER2-positive metastatic breast cancer who responded to standard induction therapy, TUKYSA, when administered as a first-line maintenance treatment, significantly extended the time to cancer progression or death compared with placebo. Both TUKYSA and placebo were given alongside the standard maintenance regimen of Roche’s breast cancer drugs HERCEPTIN and PERJETA.
The phase III HER2CLIMB-05 trial is assessing TUKYSA compared with placebo, both given alongside first-line standard-of-care maintenance therapy (trastuzumab plus pertuzumab) following chemotherapy induction. The trial achieved its primary endpoint, showing a statistically significant and clinically meaningful improvement in progression-free survival (PFS) in the TUKYSA arm versus placebo, based on investigator assessment. The combination of TUKYSA with trastuzumab and pertuzumab was generally well tolerated, with a safety profile consistent with what is known for each therapy individually.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Lion TCR Secures Triple FDA Milestones with IND Clearance for Chronic Hepatitis B; Corstasis Ther...
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- Breast Cancer Insights: Identifying Symptoms and Protecting Your Health
- Pfizer tightens DMD trial criteria over safety concerns; Kytopen raises $30M; Eli Lilly taps Reif...
- HR+/HER2- Breast Cancer: Unveiling the Worldwide Advances and Strategies
“HER2-positive breast cancer remains a difficult subtype to treat, as many patients see their disease advance even after effective first-line therapies,” said Erika Hamilton, M.D., principal investigator of HER2CLIMB-05 and Director of Breast Cancer Research at Sarah Cannon Research Institute (SCRI). “The findings from HER2CLIMB-05 suggest that incorporating TUKYSA into first-line maintenance therapy could further reduce the risk of disease progression or death, while maintaining a treatment with a well-known safety profile.”
HER2 is overexpressed in approximately 15–20% of breast cancers and is linked to poorer outcomes, with a five-year survival rate for HER2-positive metastatic breast cancer estimated at 41–47%, depending on hormone receptor status. As per DelveInsight analysis, in the US, there were nearly 45,400 incident cases of HER2-positive breast cancer in 2024. These cases are expected to rise significantly by 2034.
First-line maintenance therapy for HER2-positive metastatic breast cancer has not changed since 2012, and most HER2-positive MBC patients experience disease progression within two years of starting treatment. Until recently, treatment options for these patients have been limited. Data from HER2CLIMB-05 will be shared at an upcoming medical congress and with regulatory authorities.
“Pfizer is committed to advancing the future of first-line treatment for HER2-positive metastatic breast cancer, particularly exploring the potential for a chemotherapy-free maintenance option,” said Johanna Bendell, M.D., Chief Development Officer, Oncology, Pfizer. “The encouraging outcomes from HER2CLIMB-05, together with TUKYSA’s established safety in later-line therapies, highlight its promise in front-line maintenance, potentially benefiting a wider group of patients with HER2-positive disease. We sincerely thank the patients and investigators who made this crucial research possible.”
Since its initial approval in 2020, TUKYSA has become a standard treatment option for HER2-positive MBC in the third-line setting and is approved in over 50 countries. At the same time, certain patients might see new treatment options following positive results from last year’s phase III Patina trial, reported by the cancer research group Alliance Foundation Trials. The study found that adding Pfizer’s CDK4/6 inhibitor Ibrance to standard anti-HER2 and endocrine therapy lowered the risk of disease progression or death by 26% in patients with HR-positive, HER2-positive breast cancer who had already responded to induction therapy.
In the US, the FDA has approved TUKYSA in combination with trastuzumab and capecitabine for adult patients with advanced, unresectable, or metastatic HER2-positive breast cancer, including those with brain metastases, who have received one or more prior anti-HER2 regimens in the metastatic setting. TUKYSA is not currently approved for first-line use.
The HER2CLIMB-05 trial now shows potential to transform the broader HER2+ breast cancer treatment landscape, particularly for patients with HR-negative tumors, who represent roughly 70% of cases. The HER2+ breast cancer market has become increasingly complex, largely due to the emergence of antibody-drug conjugates, notably AstraZeneca’s and Daiichi Sankyo’s ENHERTU. At the June American Society of Clinical Oncology annual meeting, the two companies presented phase III data demonstrating that a combination of ENHERTU and PERJETA improved progression-free survival by 44% compared with the standard HERCEPTIN-PERJETA-chemotherapy regimen in first-line HER2+ breast cancer.
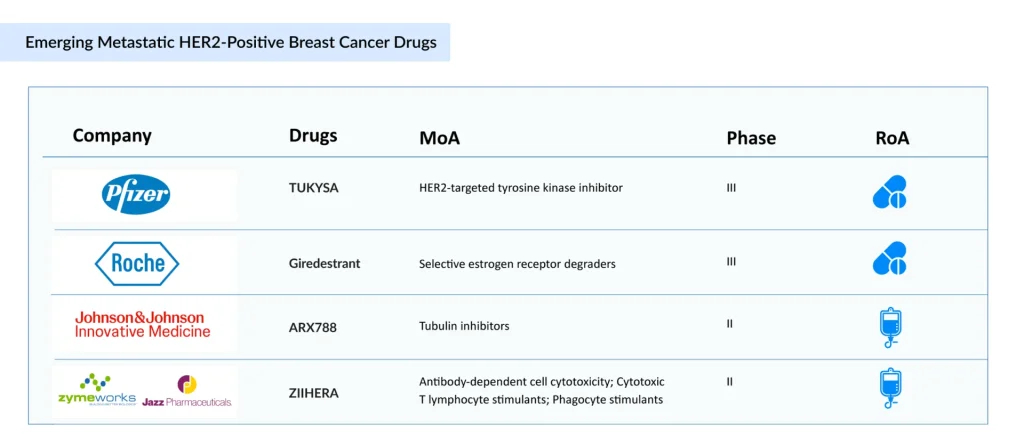
Johnson & Johnson Innovative Medicine’s ARX788, another anti-HER2 ADC, is being extensively studied for the treatment of breast cancer. It is a homogeneous and highly stable molecule that targets the HER2 receptor and carries two AS269 cytotoxic payloads, site-specifically linked to a trastuzumab-derived antibody. The therapy is currently in a Phase II clinical trial (NCT04829604, ACE-Breast-03) for HER2-positive breast cancer. In addition, a Phase III pivotal study (Breast-02) underway in China for patients with locally advanced or metastatic HER2-positive breast cancer has achieved its pre-defined interim primary efficacy endpoint with statistical significance.
Zymeworks, in collaboration with Jazz Pharmaceuticals, is working on Zanidatamab zovodotin, a HER2-targeted bispecific ADC created using Zymeworks’ proprietary Azymetric and ZymeLink platform technologies. It integrates the distinctive bispecific design of zanidatamab with a potent in-house–developed cytotoxic payload. The therapy is currently under evaluation in a Phase II clinical trial for the treatment of HER2-positive breast cancer.
Apart from these companies, Roche is working on its SERD candidate Giredestrant. Giredestrant is designed to bind to the estrogen receptor to limit its function. In addition, when SERDs bind to the estrogen receptor, they may alter the receptor’s shape, leading the cell to eliminate it through degradation. Currently, the company is conducting a Phase III trial in combination with PHESGO for 1L ER-positive/HER2-positive breast cancer, and its filing is anticipated by 2027 and beyond.
In conclusion, the competitive HER2+ space is expected to expand rapidly as next-generation ADCs, bispecific antibodies, and novel kinase inhibitors continue to mature. Agents such as zanidatamab zovodotin, and giredestrant represent the next wave of innovation focused on multi-targeted mechanisms, receptor degradation, and enhanced payload delivery.
With more agents moving into earlier lines of therapy, sequencing and resistance management will become central challenges, pushing researchers to explore rational combinations that can delay disease progression and overcome acquired resistance mechanisms. The approval of TUKYSA for first-line maintenance could catalyze a paradigm shift toward chronic, manageable therapy in HER2-positive breast cancer, transforming it from an aggressive, relapsing condition into one with durable control and improved quality of life for patients worldwide.

Downloads
Article in PDF
Recent Articles
- FDA Approves AstraZeneca’s Enhertu; Bayer Wins FDA Approval for Prostate Cancer Therapy, Nubeqa; ...
- Bayer’s New Cardiology Drug Acoramidis; Two Datopotamab Deruxtecan Applications Validated in the ...
- Ipsen to Buy Epizyme; BioMarin’s Gene Therapy for Hemophilia; AbbVie’s Qulipta for Ch...
- How HR+/ HER2-Breast Cancer Emerging Drugs Will Transform The Market?
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy