FcRn Inhibitors for Autoimmune Disorders: A Promising Therapeutic Approach
May 06, 2024
Table of Contents
Autoimmune disorders, a group of conditions where the immune system mistakenly attacks healthy cells, affect millions worldwide, often leading to debilitating symptoms and reduced quality of life. Traditional treatments for autoimmune disorders have primarily focused on suppressing the immune system, which can leave patients vulnerable to infections and other complications. However, recent advancements in medical research have opened up new avenues for targeted therapies, including the promising field of FcRn inhibitors.
Understanding FcRn: Guardians of Immunoglobulins
FcRn, or neonatal Fc receptor, plays a crucial role in the immune system by regulating the levels of immunoglobulins, also known as antibodies, in the body. These antibodies are key players in the immune response, helping to identify and neutralize harmful pathogens such as bacteria and viruses. Additionally, antibodies play a role in autoimmune disorders, where they can target the body’s own tissues, leading to inflammation and tissue damage.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Expanding Market of Complement Inhibitors
- Ceribell raises $35M; Lyra reels $29.5M; Minoryx raises Euro21M; Alexion acquired Syntimmune
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Graves’ Disease Treatment Revolution: What’s Next in Line?
- Agios’ PYRUKYND SNDA Accepted by FDA for Thalassemia; BridgeBio’s BBO-8520 Gets FDA Fast Track fo...
At its core, FcRn acts as a liaison between IgG molecules and the cellular environment. Structurally, it consists of two subunits: a transmembrane heavy chain and a soluble light chain. This unique architecture enables FcRn to bind to IgG in a pH-dependent manner, exhibiting a robust affinity under acidic conditions, such as those found in endosomes.
The journey of IgG begins upon its endocytic uptake into cells. Within the acidic milieu of endosomes, FcRn binds to IgG, forming a complex that shields the antibody from lysosomal degradation. This process, termed “rescue,” prolongs the half-life of IgG by diverting it away from the degradative pathway, thereby enhancing its availability for immune defense.
Furthermore, FcRn facilitates the transcytosis of IgG across epithelial barriers, notably in the neonatal gut and placenta. By shuttling maternal IgG to the developing fetus or neonate, FcRn confers passive immunity during critical stages of development, bolstering the infant’s defense against pathogens until its own immune system matures.
Targeting FcRn: A New Approach to Autoimmune Therapy
Targeting FcRn presents a promising avenue in autoimmune therapy, offering a novel strategy to manage autoimmune disorders. FcRn plays a crucial role in the recycling and maintenance of immunoglobulin G (IgG) levels in the bloodstream, thereby influencing immune responses. By selectively inhibiting FcRn, researchers aim to modulate IgG homeostasis, mitigating autoimmune reactions. This approach holds significant potential for various autoimmune diseases where aberrant IgG production and immune dysregulation are hallmarks, offering a precise and targeted therapeutic intervention that could improve patient outcomes and quality of life.
The exploration of FcRn targeting as a therapeutic strategy marks a paradigm shift in autoimmune therapy, moving beyond conventional treatments that often come with significant side effects and limited efficacy. By directly addressing the underlying mechanisms of autoimmune pathogenesis, FcRn-targeted therapies offer the prospect of tailored interventions with potentially fewer adverse effects. Moreover, the versatility of this approach allows for its application across a spectrum of autoimmune conditions, from rheumatoid arthritis to lupus, ushering in a new era of precision medicine where treatments are designed to specifically address the molecular pathways driving autoimmune responses.
Approved FcRn Inhibitors in the Market
Severe autoimmune disorders, marked by increased levels of harmful IgG antibodies, pose a significant medical challenge. Current therapies frequently lack effectiveness in adequately handling disorders like myasthenia gravis, warm autoimmune hemolytic anemia (wAIHA), and idiopathic thrombocytopenic purpura (ITP). Therefore, there’s an urgent need for innovative treatments that can comprehensively address these conditions.
VYVGART (efgartigimod) marked the first approval within the FcRn class of medications. This antibody fragment binds to FcRn, halting its recycling of IgG into the bloodstream. This action leads to an overall reduction in IgG levels, including the abnormal AChR antibodies associated with myasthenia gravis. The FDA granted Fast Track and Orphan Drug Designations to VYVGART. In December 2021, the FDA approved VYVGART, developed by Argenx, for treating gMG. Additionally, VYVGART is under assessment for various other conditions such as Post-COVID Postural Orthostatic Tachycardia Syndrome (PC-POTS), bullous pemphigoid, primary immune thrombocytopenia, and CIPD, among others.
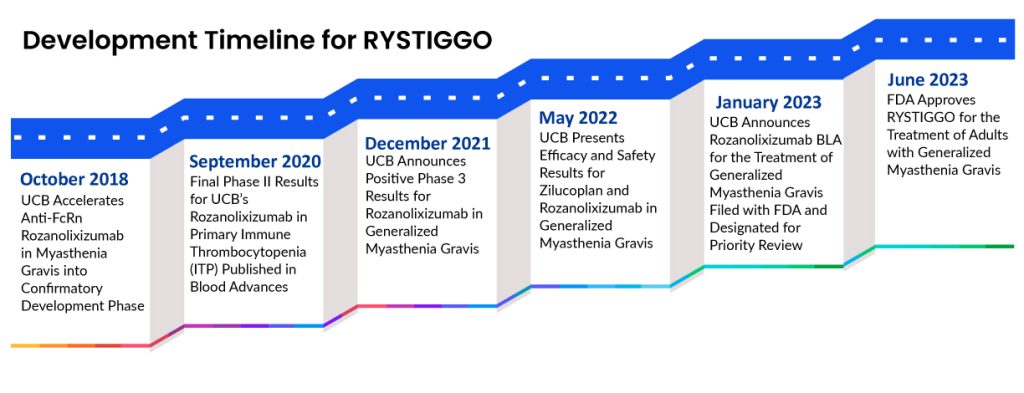
UCB Pharma developed RYSTIGGO (rozanolixizumab) for addressing autoimmune illnesses. It gained its initial approval in June 2023 in the US for managing gMG in adults with either anti-AChR or anti-MuSK antibodies. This marked the first approval in the US for gMG with both types of antibodies. Following this, in September 2023, UCB announced that RYSTIGGO was approved by the Japanese MHLW for treating adults with generalized Myasthenia Gravis, specifically for those who do not respond adequately to steroids or other immunosuppressants. Then, in January 2024, the European Commission (EC) granted marketing authorization for RYSTIGGO as an additional treatment alongside standard therapy for gMG in adult patients with either AChR or MuSK antibodies. UCB’s pipeline also includes evaluations of RYSTIGGO for MOG antibody disease, autoimmune encephalitis, and severe fibromyalgia syndrome.
As the field of FcRn inhibitors becomes increasingly competitive, it is interesting to draw comparisons between UCB’s RYSTIGGO and Argenx’s VYVGART. Both medications are advancing in comparable conditions like gMG and CIDP, highlighting the expanding possibilities and intense rivalry within this area.
The Promise of FcRn Inhibitors: Clinical Pipeline
Clinical trials investigating FcRn inhibitors have shown promising results in a variety of autoimmune disorders, including rheumatoid arthritis, systemic lupus erythematosus, and myasthenia gravis. In these studies, FcRn inhibitors have demonstrated efficacy in reducing disease activity, improving symptoms, and even inducing remission in some patients. Some of the FcRn inhibitors in the pipeline include Nipocalimab (Johnson & Johnson) Batoclimab (Immunovant), and IMVT-1402 (Immunovant), among others.
Nipocalimab, developed by Johnson & Johnson, is an experimental, highly potent, fully human, lacking sugar molecules, a non-activating monoclonal antibody designed to specifically inhibit FcRn. Its goal is to decrease the levels of various immunoglobulin G (IgG) antibodies circulating in the body, which include both self-targeting (autoantibodies) and foreign-targeting (alloantibodies) antibodies associated with numerous conditions. Nipocalimab stands out as the sole anti-FcRn treatment being investigated in three significant areas within the realm of autoantibody-related disorders: Rare Autoantibody diseases (such as generalized myasthenia gravis in adults and children, chronic inflammatory demyelinating polyneuropathy, warm autoimmune hemolytic anemia, and idiopathic inflammatory myopathies); Maternal Fetal diseases caused by maternal alloantibodies (like HDFN); and Common Rheumatology disorders (such as rheumatoid arthritis, Sjögren’s disease, and systemic lupus erythematosus). In February 2024, the FDA provided Breakthrough Therapy Designation (BTD) for nipocalimab’s use in treating pregnant individuals sensitized to foreign antigens, who are at a high risk of severe hemolytic disease of the fetus and newborn (HDFN). This designation was granted based on findings from the Phase II UNITY clinical trial focused on HDFN.
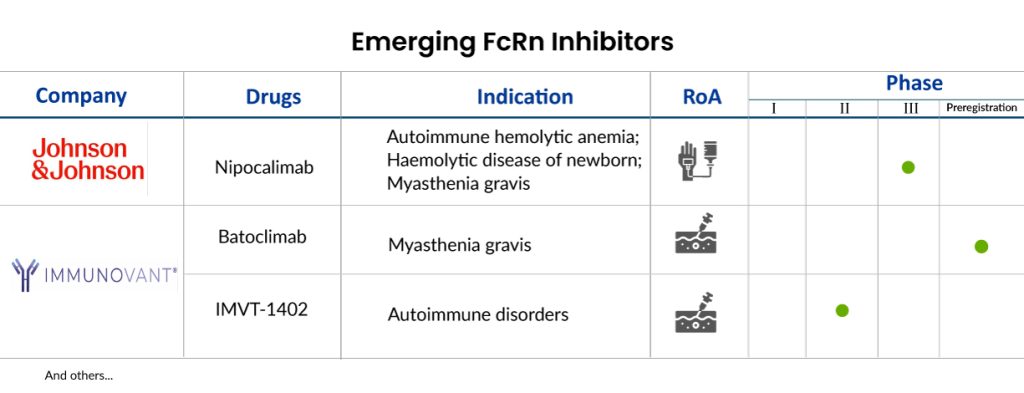
Batoclimab, a completely human anti-FcRn monoclonal antibody, inhibits FcRn-IgG interactions, hastening the breakdown of autoantibodies and offering a potential treatment for IgG-mediated autoimmune diseases. In December 2023, Immunovant reported that results from the first group of patients in an ongoing 24-week Phase II clinical trial of batoclimab for Graves’ disease showed response rates exceeding 50% significantly. The global Phase III clinical trials for batoclimab in myasthenia gravis and thyroid eye disease are advancing as planned, with expected top-line data to be released in the second half of 2024 for myasthenia gravis and the first half of 2025 for thyroid eye disease.
In addition, Immunovant has received approval from the FDA for IMVT-1402, aiming to be a leading anti-FcRn antibody for treating IgG-mediated autoimmune conditions. In early Phase I trials with healthy adults, IMVT-1402 showed promising safety and pharmacodynamic results. These qualities, along with its easy administration method allowing potential self-treatment, position IMVT-1402 as a hopeful solution for various autoimmune disorders with unmet patient needs. The company intends to kick off 4-5 programs seeking approval for IMVT-1402 by March 31, 2025 and aims to start trials for IMVT-1402 in 10 different conditions within the following two fiscal years, including the 4-5 programs by March 31, 2026.
The anticipated launch of these emerging therapies are poised to transform the FcRN inhibitors market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the FcRN inhibitors market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Challenges and Future Directions in the FcRn Inhibitors Market
While FcRn inhibitors hold great promise for the treatment of autoimmune disorders, several challenges remain to be addressed. One significant hurdle is the need for further research to optimize the efficacy and safety of these therapies, particularly in long-term use and in specific patient populations. Additionally, the high cost of biologic therapies, including FcRn inhibitors, may limit access for some patients, highlighting the importance of continued efforts to reduce healthcare disparities and improve affordability.
However, the FcRn inhibitor market has witnessed significant growth and attention, fueled by its potential to address various autoimmune diseases and related conditions. With a large addressable patient base across the 7MM and a promising pipeline of candidates like efgartigimod, the market is poised for substantial growth.
The enriching drug pipeline of FcRn inhibitors holds significant potential for large-scale companies to acquire substantial FcRn inhibitor market share. The anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to further drive market growth in the 7MM.
Looking ahead, ongoing research into the role of FcRn in autoimmune disorders and the development of novel therapeutic agents hold the potential to revolutionize the treatment landscape for these conditions. By harnessing the power of FcRn inhibition, we may unlock new possibilities for managing autoimmune disorders and improving the lives of millions of affected individuals worldwide.

Downloads
Article in PDF
Recent Articles
- CAR-T Beyond Cancer: Resetting Immunity in Autoimmune Diseases
- Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bri...
- Ceribell raises $35M; Lyra reels $29.5M; Minoryx raises Euro21M; Alexion acquired Syntimmune
- Incyte’s Clinical Trial for Myelofibrosis; Eisai and Bioge’s Leqembi; FDA Approves Reata Pharmace...
- Eisai Announces Solo Development of Farletuzumab Ecteribulin (FZEC); Johnson & Johnson’s Nipo...



