Fewer Sleepless Nights Over, Drugs for Insomnia Treatment
Jul 28, 2023
Table of Contents
“Sleep is a fundamental pillar of our overall health and well-being, and a continued lack of sleep can significantly impact many aspects of daily life”.
Insomnia and its symptoms
Insomnia is a common sleep-wake disorder where one has difficulty initiating or maintaining sleep or dissatisfaction with sleep quality or duration. It is characterized by both nocturnal and diurnal symptoms that lead to impaired quality of life.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Rise of Energy Drinks: Power in a Can or a Health Hazard?
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- GSK’s Covifenz; Idorsia’s Quviviq; GSK’s ZEJULA; EMA Expands its Nod for BMS, Eli Lilly, an...
- AstraZeneca’s Farxiga; Incyte’s Jakavi; FDA Fast Track Status to HM43239 for R/R AML; Idorsia’s I...
- Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
Insomnia is bi-directional and may occur along with psychiatric or physical conditions. The common insomnia symptoms include difficulties initiating sleep at bedtime, frequent or early awakenings with an inability to return to sleep, fatigue, mental impairment, impaired social or academic performance, mood disturbances, daytime sleepiness, behavioral problems (e.g. hyperactivity, impulsivity, and aggression), reduced motivation/energy, and dissatisfaction with sleep.
Stress, irregular sleep schedules, mental health disorders (anxiety and depression), physical illnesses and pain, medications, neurological problems, and specific sleep disorders (restless leg syndrome, narcolepsy) are common causes of insomnia.
Insomnia diagnosis and the attached stigma
Insomnia is clinically diagnosed when the impact on sleep quantity or quality is persistent for a minimum of three months (at least three nights per week) and occurs despite an adequate opportunity to sleep, with adverse effects on daytime functioning.
The diagnosis of insomnia has evolved with time and has changed with each edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM). With the introduction of DSM-V criteria, the diagnostic criteria removed the distinction between primary and secondary insomnia, and insomnia associated with comorbid conditions was considered. These changes have improved the diagnosis of insomnia. They have helped in the recognition of insomnia as a disorder in its own right, along with greater operationalization and quantification of its diagnostic criteria.
There have also been considerable improvements in the monitoring of sleep remotely with smartwatches, fitness trackers, and other consumer wearables in the last few years, with the sleep-tech market burgeoning with new entrants and impressive innovations. Despite this, and perhaps due to the presence of stigma around insomnia as a disorder, there is a lack of sufficient conversation between patients and their doctors around the importance of quality sleep resulting in far-reaching implications on patients’ overall health.
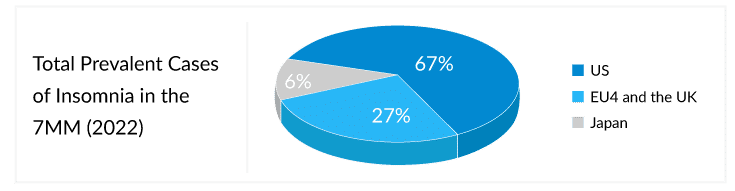
According to DelveInsight’s estimates, in the 7MM, the total diagnosed prevalent cases of insomnia were approximately 86 million in 2022, of which the US accounted for 67% of the cases.
According to ICSD-III, insomnia is now sub-classified as short-term, chronic, or other. The previous sub-classification of primary or comorbid was eliminated as it did not improve diagnostic accuracy or differentiate insomnia treatment options.
Acute insomnia also known as short-term or adjustment insomnia lasts from one night to a few weeks. It’s the most common type of insomnia and typically occurs due to a stressful event.
Chronic insomnia disorder is a persistent medical condition, where an individual has trouble sleeping for at least three nights per week for at least three months, and occurs despite an adequate opportunity to sleep. A key symptom of chronic insomnia is the impairment of daytime functioning, which impacts the day to day life.
The US has a higher prevalence of acute insomnia, and due to improved diagnosis and awareness, more than half of these individuals recover without developing persistent poor sleep or chronic insomnia. However, in Europe, a higher prevalence of chronic insomnia is seen as patients are underdiagnosed or poorly managed due to a lack of awareness, regional disparities in practices, and access to healthcare services.
Current insomnia treatment market paradigm
The treatment goal for insomnia is to improve the patient’s ability to fall asleep, stay asleep, wake and function well. As per various guidelines, insomnia is primarily treated using behavioral and psychological therapies such as Cognitive Behavioral Therapy for insomnia (CBT-I).
CBT-I is a multi-component, gold standard for treating chronic insomnia, including cognitive, behavioral, and psychoeducational interventions. It is the first line of treatment recommended for insomnia and is delivered through 4–8 sessions, typically by a clinician with specialized training in this area. Though effective in improving sleep outcomes such as sleep time, efficiency, and onset latency, there are barriers that prevent patients from engaging with CBT-I treatment. Some of these include lack of access to trained sleep therapists, cost restraints, insurance coverage, patient unwillingness to participate actively, and patient adherence issues. To overcome such barriers, new digital CBT-I (dCBTI) are emerging. These are considered part of the Consumer Sleep Technology (CST) and may or may not need FDA approval based on whether their claims are directed toward wellness benefits or clinical benefits. Pear Therapeutics’, SOMRYST, is one such dCBTI which is the first and only digital therapeutic drug to be FDA-approved to treat chronic insomnia.
Over the years, various drug classes have been used for insomnia treatment, these include benzodiazepines (temazepam, triazolam, estazolam, flurazepam, and quazepam), non-benzodiazepines (also called “Z-drugs”) (zolpidem, eszopiclone, zaleplon, or zolpidem tartrate), both of which are usually used as first-line pharmacotherapy. The additional classes approved by the US FDA include selective histamines antagonists, melatonin receptor agonists, such as ramelteon, and orexin receptor antagonists, such as suvorexant, lemborexant, and daridorexant.
Benzodiazepines, promote sleep and reduce anxiety by binding to gamma-aminobutyric acid-A (GABA-A) receptors in the brain, low levels of which lead to trouble falling asleep. But since these have a risk for dependency, they are not recommended for long-term use. The Z-drugs have a similar hypnotic effect as benzodiazepines, as they also raise the GABA levels in the brain. Though more efficacious than BZDs, these are also associated with some significant side effects, including drowsiness the next morning, impacting quality of life.
Melatonin receptor agonists, like Ramelteon which is approved for insomnia treatment characterized by difficulty falling asleep, selectively bind to the MT1 and MT2 melatonin receptors in the brain. They work to create a manageable circadian rhythm or sleep-wake cycle. This leads to an increase in the release of the sleep-promoting hormone, melatonin, which helps individuals fall asleep easily. Though helpful in falling asleep initially, these aren’t recommended for those who wake up in the middle of the night and have trouble getting back to sleep. Further, even though they are considered safe than benzodiazepines and Z-drugs, they can still cause daytime drowsiness and affect coordination.
While most of these drug classes have been on the insomnia treatment market for a while, drugs with novel MOAs have been awaited.
The dual orexin receptor antagonists (DORAs), are relatively new entrants in the insomnia treatment market. DORAs prevent one from feeling awake at night, by reducing wakefulness and increasing sleep duration, instead of resetting the biological clock or inducing sleep. These decrease the production of orexin in the brain, thus blocking wakefulness.
Although considered safer than other classes, they do come with risks and potential side effects (these may cause daytime drowsiness the next day). The drugs in this class are also more expensive compared to others for insomnia. Their market growth has not been as one would have anticipated, even though the class has three approved products, BELSOMRA, DAYVIGO, and QUVIVIQ already. According to estimates based on DelveInsight’s model, the DORA class captured only about 6 % of the total insomnia market (around USD 6,000 million), in the 7MM, in 2022.

Merck’s BELSOMRA (suvorexant), the first entrant in the US (2014) market has failed to catch on with patients despite being in the market for nearly seven years. Its first-to-market advantage hasn’t translated into large annual sales and has stayed lodged below the USD 100 million mark since 2019. Eisai’s DAYVIGO (lemborexant), has also struggled to gain traction with lackluster sales in the three quarters of the last fiscal year. Both insomnia drugs have faced difficulties in gaining market share, and the warning labels regarding next-day residual effects and abuse potential, among other safety risks, have added to the ill fortune.
The latest DORA in the US and Europe insomnia treatment market is Idorsia, Syneos Health, and Mochida Pharmaceutical’s QUVIVIQ (daridorexant). The drug aced phase III trials, establishing that it not only assists in falling and staying asleep but also reduces daytime sleepiness due to its shorter half-life, setting it apart from the other marketed DORAs.
Further, as BELSOMRA and DAYVIGO could not make inroads in the EU, QUVIVIQ’s approval in the region for chronic insomnia might keep Idorsia ahead of rivals, Merck and Eisai, providing it a chance for a breakthrough in the region. Even in the US, QUVIVIQ has attained a feat not accomplished in the past. The drug will have improved access as Express Scripts and CVS insurance plans cover it. Regional plans like TRICARE, and healthcare programs for active and retired US military personnel and their families will also ensure its accessibility.
To grow in the space, Idorsia has also initiated a series of wide-ranging premarketing “edutainment”, outreach, and awareness campaigns, leveraging social media and other digital and online channels to amp up awareness. It has also started the “Seize the Night & Day” campaign. QUVIVIQ is clearly more likely to establish a dominant foothold in the DORA treatment market than its predecessors and plans to seek approval in the Japan market as well.
The future outlook of the insomnia treatment market
Insomnia is a ubiquitous disease with unique predisposing factors and may require long-term treatment that is cost-effective and has limited side effects. Despite the advances in insomnia treatment modalities over the last few years, there are various unmet needs still to be addressed:
- There is a lack of clinical evidence of the risks and benefits of the approved therapies to guide clinical decision-making. This leads to prescribing of off-label antidepressants and other OTCs.
- Insomnia is a heterogeneous disease and patients may require personalized treatment management. The vast majority of studies that evaluate insomnia may not correlate with secondary causes. Trials are needed to compare effective treatments to match specific patient types.
- Most existing pharmacological treatments are associated with adverse events, like reduced sleep quality, next-day somnolence, and increased risk of accidents/injuries
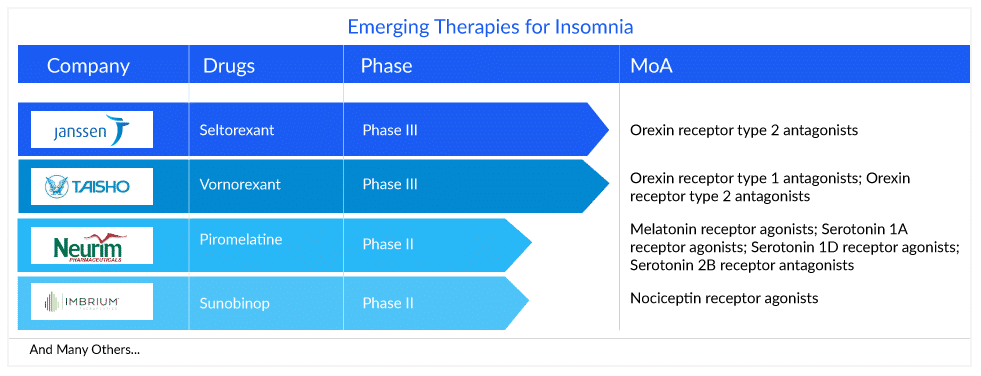
Various insomnia drugs are being developed, like vornorexant, piromelatine, seltorexant, and sunobinop, which may potentially address some of these concerns. The insomnia treatment market will see some movement during the forecast period (2023–2032) as new therapies are likely to hit the market and compete with the already generalized existing classes and OTC drugs.
Janssen’s seltorexant (JNJ-42847922), a selective orexin receptor antagonist (SORAs), is a novel drug, being developed as an adjunctive therapy in major depressive disorder with insomnia symptoms. The drug selectively blocks the orexin-2 receptors, while leaving the orexin-1 receptors unaffected. It has the potential to offer a new insomnia treatment option for patients who have not responded to currently available therapies, especially those with comorbidities, such as anxiety or depression.
Vanda Pharmaceuticals’ HETLIOZ (tasimelteon), a melatonin receptor agonist, is another therapy that is anticipated to enter the US insomnia treatment market by 2024, according to DelveInsight’s analysts. This circadian regulator demonstrated excellent Phase II results wherein it improved latency to persistent sleep significantly and is expected to become a blockbuster product with projected revenue of more than USD 1000 million by 2032 in the 7MM.
Other products in the insomnia pipeline with potential are Vornorexant (TS-142) (Taisho Pharmaceutical), Sunobinop (IMB-115) (Imbrium Therapeutics), Piromelatine (Neu-P11) (Neurim Pharmaceutical), EVT201 (Evotec/ JingXin), etc.
While the drugs in the insomnia pipeline are not out of the box, they are still awaited with optimism to mend many broken sleepless nights and address the very complex nature of insomnia. Their triumphs with effectiveness, outreach, and insomnia treatment market capture only time will tell.
FAQs
Insomnia is a common sleep-wake disorder where one has difficulty initiating or maintaining sleep or dissatisfaction with sleep quality or duration. It is characterized by both nocturnal and diurnal symptoms that lead to impaired quality of life.
The common insomnia symptoms include difficulties initiating sleep at bedtime, frequent or early awakenings with an inability to return to sleep, fatigue, mental impairment, impaired social or academic performance, mood disturbances, daytime sleepiness, behavioral problems (e.g. hyperactivity, impulsivity, and aggression), reduced motivation/energy, and dissatisfaction with sleep.
Insomnia is diagnosed based on the subjective complaint of difficulty initiating or maintaining sleep and reports of significant distress or daytime impairments. Actigraphy and polysomnography are currently used to measure sleep activity objectively. Although polysomnography is the gold standard for measuring sleep disorders, it is not commonly used to diagnose insomnia. Insomnia questionnaires such as the Insomnia Severity Index (ISI) and the Pittsburgh Sleep Quality Index (PSQI) are useful for insomnia diagnosis.
The goal of insomnia therapy is to improve the patient’s ability to fall asleep, stay asleep, wake up, and function normally. Insomnia is primarily treated with behavioral and psychological therapies such as CBT-I, according to various guidelines. To treat the condition, various pharmacological therapies, such as benzodiazepines or a combination of psychologic and pharmacologic therapies, are recommended.

Downloads
Article in PDF
Recent Articles
- Sleep Disorders: An Emerging Public Health Issue
- The Rise of Energy Drinks: Power in a Can or a Health Hazard?
- GSK’s Covifenz; Idorsia’s Quviviq; GSK’s ZEJULA; EMA Expands its Nod for BMS, Eli Lilly, an...
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- AstraZeneca’s Farxiga; Incyte’s Jakavi; FDA Fast Track Status to HM43239 for R/R AML; Idorsia’s I...