Novartis’ LUTATHERA for GEP-NET Treatment: Ray of Hope for Pediatric Patients
Apr 26, 2024
Table of Contents
2024 could be an eventful year for LUTATHERA and radioligand therapies (RLTs) around their future commercial prospects.
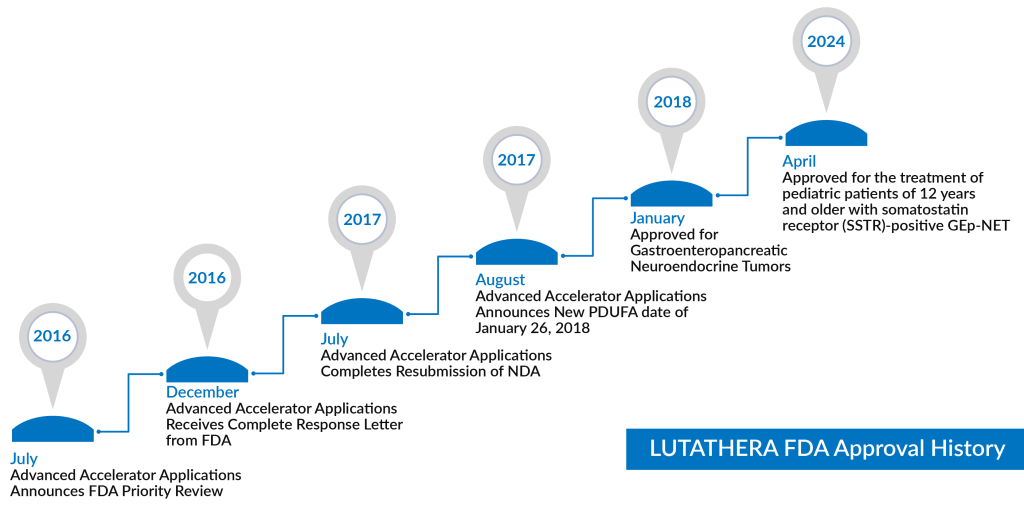
On April 23, 2024, the FDA approved LUTATHERA (lutetium Lu 177 dotatate) for the treatment of pediatric patients aged 12 years and older with somatostatin receptor (SSTR)-positive gastro-entero-pancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors. Somatostatin receptor type 2 (SSTR2) is expressed in 80-90% of GEP-NET tumors. LUTATHERA has previously received approval in 2018 for the treatment of GEP-NETs in adult patients. This recent approval was based on pharmacokinetic (PK), dosimetry, and safety data from the NETTER-P study, which showed that the safety profile of lutetium Lu 177 dotatate in pediatric patients aged 12 to 17 was consistent with that observed in adult patients. This application was granted priority review and orphan drug designation. By leveraging the potency of radioactive atoms and directing them toward advanced cancer cells, radioligand therapy (RLT) theoretically enables precise radiation delivery to targeted cells throughout the body, offering a promising avenue for neuroendocrine tumor treatments. This approach aims to address the challenging symptoms associated with neuroendocrine tumor cancer, including those related to pancreatic neuroendocrine tumors and other neuroendocrine tumor symptoms.
Downloads
Click Here To Get the Article in PDF
The latest approval added another feather in the cap for LUTATHERA as it is now approved in all settings of GEP-NET i.e. refractory, front-line, adults, and pediatrics/adolescent groups.
What Does it Mean for Novartis?
As for the year 2023, LUTATHERA generated sales of $605 million, 28% higher than that from 2022. With this additional approval, DelveInsight’s analysts expect a blockbuster status in the coming years for LUTATHERA. In terms of pricing, the company has opted for a value-based pricing model. The list price (WAC) of LUTATHERA is $55,896, and most patients require about four doses. The dosage is the same for adults and adolescents.
Expanded LUTATHERA Treatment Options
LUTATHERA becomes the first therapy specifically approved for use in pediatric patients with GEP-NETs. This approval addresses a critical need for new treatment options for these vulnerable patients. The drug’s efficacy and safety profile in adults have already been established, and now its availability for pediatric patients broadens the therapeutic landscape. The label expansion will further push LUTATHERA’s market share in GEP-NET.
GEP-NET Patient Burden to Drive the Market Growth
The patient burden of GEP-NETs has been increasing over the past several decades, with steadily rising incident rates in North America, Asia, and Europe. The most significant rise is observed in the United States, where more than 12,000 people in the United States are diagnosed with a form of NET each year.
The global GEP-NET therapeutics market is further expected to increase by the major drivers such as rising incident population, technological advancements, increased funding for new drug development and clinical trials, increasing awareness among common people, increasing research activities and upcoming therapies in the forecast period. As awareness grows and more patients are diagnosed, demand for effective therapies will rise, potentially driving market growth.
Radioligand Therapy Advancements
Radioligand therapy (RLT) represents a promising frontier in the realm of targeted cancer treatment, leveraging the specific binding properties of radiolabeled molecules to deliver precise doses of radiation directly to cancer cells. In this therapeutic approach, a radioactive substance, known as a radioligand, is attached to a targeting molecule, such as an antibody or a small molecule that binds to receptors or antigens on cancer cells. Once administered, these radioligands seek out and bind to their targets, delivering localized radiation that selectively destroys cancer cells while minimizing damage to healthy tissues.
RLT holds great potential for treating various types of cancer, offering a personalized and effective treatment option for patients who may not respond to traditional therapies. As research continues to advance in the field of radioligand therapy, it offers hope for improved outcomes and enhanced quality of life for cancer patients worldwide.
LUTATHERA is part of the emerging field of RLT and its approval underscores the potential of RLTs in shaping the future of cancer care. Novartis, a leader in RLT, is investigating new isotopes, ligands, and combination therapies to extend beyond GEP-NETs, and into prostate, breast, colon, lung, and pancreatic cancer treatment.
With LUTATHERA’s approval, the market for neuroendocrine tumor treatments is likely to expand, as the success of LUTATHERA may encourage further research and development in this area, leading to more innovative therapies.
Competitive Landscape in the GEP-NET Treatment Space
Novartis may have a competitive edge over other therapies because this is the first drug authorized for use in a pediatric setting. Novartis is expected to boost its market potential with this approval.
However, it is worth highlighting that Novartis faces challenges from both new rivals and expected generic entrants, therefore, the company must ramp up its strategy faster.
In January 2024, Lantheus Holdings announced that its Abbreviated New Drug Application (ANDA) for Lutetium Lu 177 Dotatate (177Lu- PNT2003), a generic version of LUTATHERA, has been accepted for filing by the FDA. According to Lantheus, it is the first applicant to submit a substantially complete ANDA for lutetium Lu 177 dotatate.
The pharmaceutical industry is becoming more interested in targeted radiation therapy as a novel, highly promising cancer treatment option as a result of the LUTATHERA success story.
In March 2024, AstraZeneca entered into a definitive agreement to acquire Fusion Pharmaceuticals, a clinical-stage biopharmaceutical company developing next-generation radioconjugates.
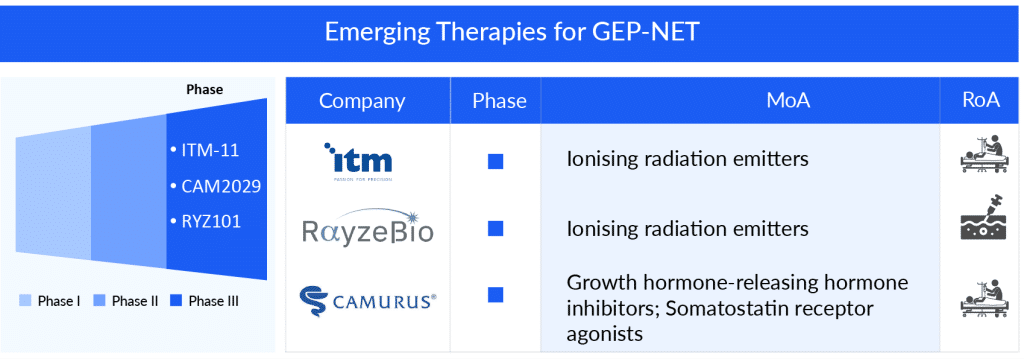
Other emerging GEP-NET drugs like RYZ101 (RayzeBio), CAM2029 (Camurus), ITM-11 (ITM Isotope Technologies Munich), Cabozantinib (Exelixis), Lurbinectedin (PharmaMar) are expected to enter the GEP-NET treatment market in the future, driving the market growth further.
Challenges and Opportunities in the GEP-NET Treatment
- While LUTATHERA’s approval is promising, challenges remain. These include accessibility, cost, and patient awareness.
- Market dynamics will depend on factors such as reimbursement policies, physician adoption, and patient education.
- Pharmaceutical companies may explore pricing strategies to balance affordability and sustainability.
What KOLs Are Talking About LUTATHERA Expanded Approval?
“LUTATHERA marks a significant milestone as it becomes the inaugural treatment sanctioned exclusively for pediatric patients grappling with GEP-NETs,” remarked Tina Deignan, Head of Oncology US. “The approval underscores the remarkable promise of radioligand therapies in revolutionizing cancer management. This approval represents a pivotal stride in advancing our dedication to exploring and advancing the RLT platform across diverse cancer categories and treatment scenarios.”
“While GEP-NETs in children and adolescents are rare, the impact can be devastating,” said Dr Theodore Laetsch, trial investigator and Director of Developmental Therapeutics Program, Children’s Hospital of Philadelphia (CHOP), a NETTER-P clinical trial site.
“The introduction of radioligand therapy significantly advanced how we treat GEP-NETs, and I’m encouraged that younger patients now have the potential to benefit from this innovation.”
“Today’s approval addresses a critical need for new treatment options for these vulnerable patients,” said Theodore Laetsch, M.D., trial investigator and director, of the Developmental Therapeutics Program, Children’s Hospital of Philadelphia (CHOP).
DelveInsight’s consultants believe LUTATHERA’s FDA approval represents a positive step toward improving outcomes for pediatric patients with GEP-NETs. Its impact on the GEP-NET market will be influenced by clinical adoption, patient needs, and ongoing research efforts.

Downloads
Article in PDF
Recent Articles
- Novartis buys Medicines; Blackstone Life Sciences and Ferring’s collaboration; Alexion̵...
- Gastroenteropancreatic Neuroendocrine Tumours Market To Gain Substantial Momentum With Entrance o...
- Rapt Therapeutics files IPO; Novartis stakes $80M for NASH
- The Business Cocktail
- Pandion bags $58M; Carmot Therapeutics grabs $15M; Innovent looks for IPO; Collaboration of Sando...