Will Merck’s P2X3 Receptor Antagonist for Chronic Refractory Cough Treatment Be Able to Turn FDA’s Nay Into Yea?
Feb 06, 2023
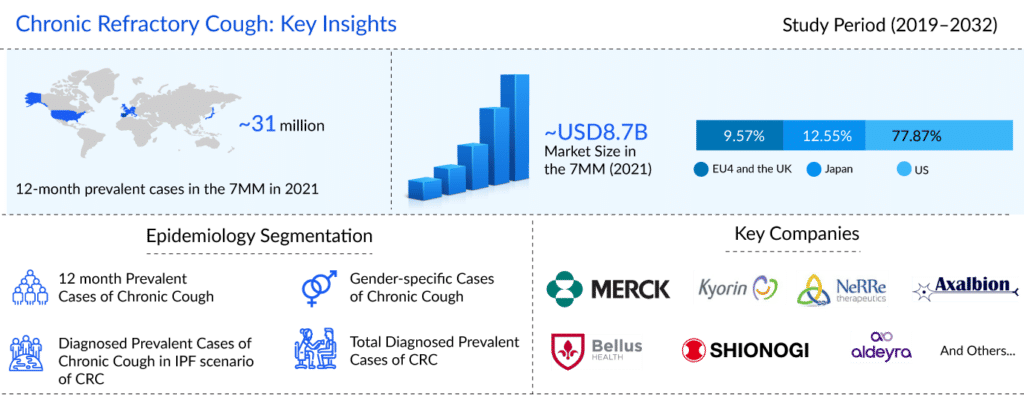
Patients who are battling this crippling ailment find that chronic refractory cough (CRC), also known as refractory chronic cough (RCC) or unexplained chronic cough (UCC), proves to be a major burden. DelveInsight’s analysis estimates that in the US, there were approximately 12.7 million 12-month prevalent episodes of chronic cough, and approximately 5.2 million of these chronic cough patients had chronic refractory cough in the year 2021. The disease often has a prior history of a viral respiratory tract infection, and the condition is typically nonproductive.
The current chronic refractory cough treatment regimen includes neuro-modulating agents, proton pump inhibitors, inhaled corticosteroids (ICS), and others. Neuromodulators include opiates (morphine, codeine, tramadol, etc.), gabapentin, pregabalin, morphine, amitriptyline, and baclofen, which act on the heightened neural sensitization that is involved in the pathogenesis of chronic refractory cough.
One of the most commonly used chronic refractory cough drugs is codeine, which is a weak opioid found naturally in poppy extracts. Gabapentin, which binds to the 2 subunit of the voltage-dependent calcium channel, is also considered a chronic refractory cough treatment option. It is typically used to treat neuropathic pain and epilepsy, but it can also be used to relieve pain during coughing in chronic refractory cough patients.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks ...
- Merck’s Remicade; Sanofi, Regeneron set Kevzara; Novartis to sever; Teva puts women’s...
- Overwhelming success of Gilead/Kite’s Yescarta; Ridgeback/ Merck in the COVID-19 game; Anap...
- Allergan sell; AZ, Merck team up; Roche’s MS drug; Chinese API maker receives a warning letter
- Limited Refractory Chronic Cough Treatment Landscape: LYFNUA Approval Restricted to Europe and Japan
Amitriptyline is a serotonin reuptake inhibitor, but it may also have effects on the muscarinic, adrenergic, and histaminergic systems. Several studies have found that treating chronic cough with amitriptyline reduces cough. Baclofen, a Gamma-Aminobutyric Acid (GABA) agonist that relieves the pain associated with coughing, is another drug that can be used for chronic refractory cough treatment. ICS, which are effective in eosinophilic airway inflammation, are another class of drugs used for chronic refractory cough treatment.
Combination therapy can also be used to treat chronic refractory cough patients. This includes combining nonpharmacologic and pharmacologic interventions, such as combining speech therapy with drugs to provide patients with relief. Patients undergoing speech therapy may be advised to take medications such as pregabalin or gabapentin. This may help with cough severity, cough frequency, and improving patients’ QoL. This can help reduce coughing to some extent.
However, these interventions though approved for chronic cough, do not treat a recurrence of cough, i.e., chronic refractory cough or unexplained chronic cough. Given that no company has received an FDA nod for CRC, the current chronic refractory cough treatment market is dominated by off-label therapies.
Due to its inexplicable nature or lack of response to any effective medication, chronic refractory cough remains a tough-to-treat indication that drastically lowers patients’ quality of life. The pharmacologic therapies that are employed cannot be used in the long term due to various adverse events associated with them, such as somnolence, dizziness, and fatigue associated with them. With limitations in current off-label chronic refractory cough treatment and no approved curative definitive therapy, the US chronic refractory cough treatment market holds a critical unmet need.
Several companies, including Merck & Co, Kyorin Pharmaceuticals, NeRRe Therapeutics, Bellus Health, Shionogi, Axalbion, Aldeyra Therapeutics, and others, are working towards bringing therapies that are more effective in reducing cough frequency, dosage, treatment duration, and quality of life than the current chronic refractory cough treatment regime.
It is believed that chronic cough results from excessive P2X3 receptor activation, which is associated with neuronal hypersensitivity in the lungs and airways. Companies view the P2X3 receptor as a possible target for chronic refractory cough treatment in the hopes that P2X3 receptor antagonists can lessen cough and prevent central nervous system side effects, including sedation, which are typical of many current antitussive medications.
In January 2022, Merck’s Gefapixant, an orally administered selective P2X3 receptor antagonist, was approved by the Japan Ministry of Health, Labor and Welfare (MHLW) for adults with RCC or UCC under the brand name LYFNUA. However, the FDA issued a CRL regarding NDA for gefapixant. Though the safety of the drug was not in question, the CRL requested additional information related to the drug’s efficacy.
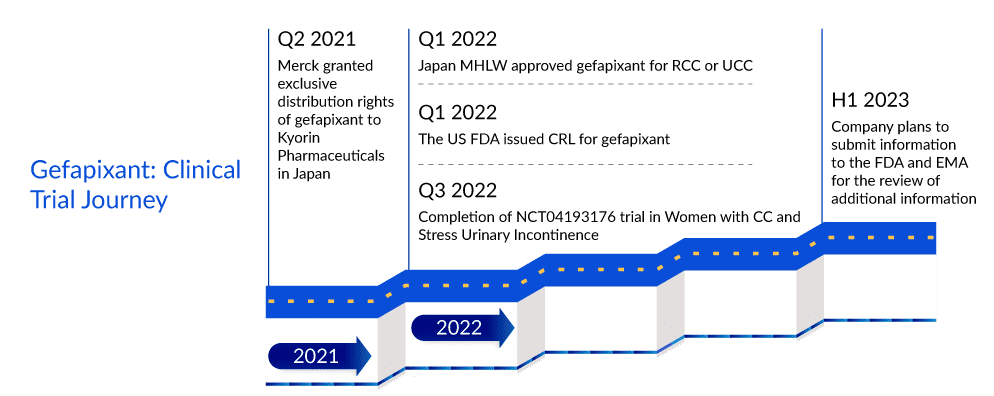
It would be interesting to see if Merck will be able to turn around the FDA’s reservations. As per DelveInsight’s analysts, even while the rejection might have given Bellus Health and other rivals more breathing time in a race to the US chronic refractory cough treatment market, it is still expected that with Merck’s commitment to advancing gefapixant for chronic refractory cough treatment, it will enter the US chronic refractory cough treatment market in 2024, a year before Bellus’ BLU-5937. The review period in the EU has also been extended pending the receipt of additional information from the Company. The Company plans to submit the information to the EMA in the first half of 2023. Even if gefapixant enters the US chronic refractory cough treatment market the earliest, one cannot turn a blind eye to BLU-5937’s superiority considering its best-in-class selectivity for P2X3. While gefapixant 45 mg twice per day showed significant reductions of 18.5% and 14·6% in 24-h cough frequency compared with placebo at week 12 and week 24 in the COUGH-1 and COUGH-2 trials, respectively, BLU-5937 in SOOTHE trial showed 53% reduction from baseline in 24-hour cough frequency at day 28 with 50 mg and 200 mg BID doses. Thus, once in the chronic refractory cough treatment market, Bellus’ drug is expected to deliver tough competition to gefapixant.

FAQs
Chronic Refractory Cough (CRC) is defined as a cough that persists for more than 8 weeks despite standard treatment and can only be treated by different medical specialties. Patients with chronic cough have a lower quality of life and their daily activities are disrupted.
Chronic refractory cough symptoms and signs are comparable to those of chronic cough. A persistent or chronic cough is one of the first and most important symptoms of respiratory involvement. Other pulmonary symptoms and signs include wheezing, stridor, dyspnea, hoarseness or aphonia, and discomfort over the laryngotracheal cartilage. One of the most common chronic refractory cough symptom is a dry, irritating cough that is limited to the laryngeal region.
Laryngopharyngeal reflux (LPR) is a common cause of CRC and may be present without the symptoms of classic GERD, such as heartburn (silent reflux). OSAS is also an independent risk factor for chronic cough and a risk factor for LPR/GERD that is resistant to medical treatment. Another common cause of chronic refractory cough is ACE inhibitors, which can occur spontaneously even after many years of use with no previous problems.
The first step in evaluating people with CRC is to perform a thorough anamnesis, followed by a physical examination. Other methods for determining the severity of CRC include functional respiratory tests, pulmonary radioscopy, a lung CT exam, or bronchoscopy with bronchopulmonary lavage.
According to ACCP guidelines, four types of treatment are available for CRC: nonpharmacologic therapies, inhaled corticosteroids, neuromodulatory therapies, and others. In January 2022, the Japan MHLW approved LYFNUA (gefapixant) for adults with RCC or UCC.
Downloads
Article in PDF
Recent Articles
- Metastatic HER2-Positive Breast Cancer Landscape: What You Need to Know
- Merck to Acquire Harpoon Therapeutics; Novo Nordisk Enters Into Collaborations with Omega Therape...
- Remdesivir accelerates for COVID-19 recovery; Merck pact with Almac; Pfizer, BioNTech finish dosi...
- Alzeimer’s drug; Guardant Health receives approval; First Patient Dosed in Phase 1 Clinical Trial...
- EC grants; Pfizer cuts; 27 medicines sold; Keytruda nabs



