HR+/HER2- Breast Cancer: Unveiling the Worldwide Advances and Strategies
Aug 08, 2024
HR+/HER2- Breast Cancer is the most prevalent form of breast cancer. This type accounts for a higher percentage among all breast cancers. Hormone receptors are proteins that receive hormone signals and cue the cancer cells to grow. If breast cancer cells get signals from the hormone estrogen that could promote tumor growth, it is known as estrogen receptor-positive (ER+) breast cancer. If cancerous cells get signals from the hormone progesterone that could promote growth, it is known as progesterone receptor-positive (PR+) breast cancer. Breast cancer that is ER-positive or PR-positive falls under the category of hormone receptor-positive (HR+) breast cancer. In addition to this, there is another factor that is also responsible for breast cancer which is known as human epidermal growth factor receptor 2 (HER2). HER2 is a gene that helps control how cells grow, divide, and repair themselves.
HR-positive cancer is usually treated with hormone therapies or a combination of hormone therapy with targeted therapy to help stop tumor growth first. However, sometimes, cancer outfoxes the treatment and becomes resistant to hormonal therapy, and stops working. There are many causes of breast cancer in women observed in comparison to men.
HR+/HER2- Breast Cancer Epidemiology

The HR+/HER2− Breast Cancer epidemiology chapter in the report provides a comprehensive analysis of historical and forecasted epidemiology, segmented by the total incident cases of breast cancer, incident cases of HR+/HER2− breast cancer, menopausal status, stage-specific cases, age-specific cases, and treatment-eligible pool in the 7MM, which includes the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, from 2020 to 2034. According to DelveInsight’s estimates, the total incident population of HR+/HER2− breast cancer in the 7MM was nearly 476,000 cases in 2023, with an expected increase during the forecast period of 2024–2034.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- GE Healthcare-Optellum’s collaboration; Ocugen’s Covid-19 vaccine trial; Blueprint to acqui...
- AbbVie & Genmab’s Collaboration; Lilly’s antisense neuro deal; COVID-19 treatment upda...
- Ovarian Cancer Treatment Drugs and Unmet Needs
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- From Lab to Bedside: The Rise of AI-Enabled Digital Pathology
Breast Cancer Statistics indicate that the HR+/HER2− breast cancer cases were highest in the United States, accounting for approximately 208,400 cases in 2023, with the majority occurring in individuals aged between 60 and 79 years, representing about 48% of the cases. Among the EU4 and the UK, Germany had the highest number of total incident cases of HR+/HER2− breast cancer, with around 50,200 cases in 2023, while Spain had the least number of incident cases. In Japan, there were approximately 43,000 treatment-eligible cases of HR+/HER2− breast cancer in 2023. These Breast Cancer Statistics highlight the prevalence and incidence trends, providing insights into how common breast cancer is and underscoring the importance of effective breast cancer treatment strategies.
HR+/HER 2- Breast Cancer Market
The HR+/HER2− breast cancer landscape is rapidly evolving, with significant market share and innovative treatments paving the way for better outcomes. In 2023, the United States dominated the market for HR+/HER2− breast cancer, capturing nearly USD 7.5 billion, a figure projected to grow significantly during the forecast period. Among the EU4 countries, Germany led the market share, while Spain had the smallest share.
The current treatment market for HR+/HER2− breast cancer includes CDK4/6 inhibitors, PARP inhibitors, SERDs, and PI3K-alpha inhibitors. Among these, IBRANCE had the largest market size in 2023, generating around USD 4.5 billion in revenue. The market size for HR+/HER2− breast cancer in the EU4 and the UK was approximately USD 2.3 billion in 2023, with expectations of continued growth from 2024 to 2034.
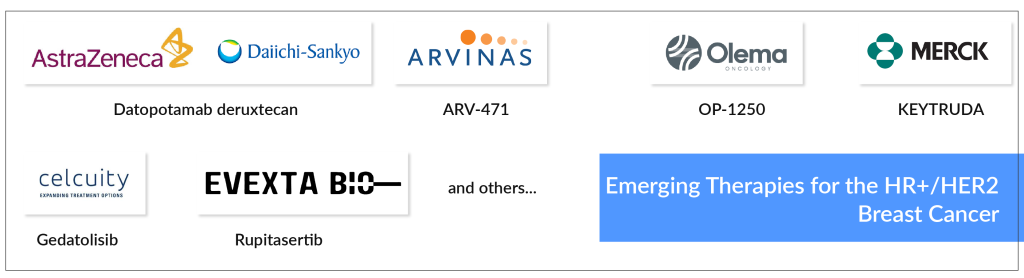
Some of the drugs in the pipeline include Datopotamab deruxtecan (AstraZeneca and Daiichi Sankyo), ARV-471 (Arvinas), OP-1250 (Olema Pharmaceuticals), KEYTRUDA (Merck), Gedatolisib (Celcuity), Rupitasertib (Evexta Bio) among others.
The uptake of these new drugs is reshaping the treatment paradigm for metastatic HR+/HER2− breast cancer. Innovative therapies such as oral SERDs, which exhibit higher bioavailability than fulvestrant, and drugs like Giredestrant, have shown higher clinical benefits and objective response rates.
The past decade has seen endocrine therapy as the preferred treatment for HR-positive and HER2− metastatic breast cancer, including cases with visceral involvement. This systemic approach, encompassing antiestrogens and aromatase inhibitors (AIs), remains the primary treatment for HR+/HER2− metastatic breast cancer due to its favorable profile. The combination of endocrine therapy with targeted agents like CDK4/6 inhibitors or mTOR inhibitors offers a promising option for patients who do not qualify for chemotherapy or sole endocrine treatment.
The addition of CDK4/6 inhibitors to endocrine drugs in the first line of treatment has significantly increased median progression-free survival (PFS). Three CDK4/6 inhibitors—palbociclib, ribociclib, and abemaciclib—have been approved for HR+/HER2− advanced or metastatic breast cancer. In Japan, these inhibitors have recently received regulatory approval, further expanding their reach. AstraZeneca and Daiichi’s ENHERTU, approved in 2022 for HER2-low breast cancer, and TRODELVY, authorized in 2023 for previously treated HR-positive, HER2-negative breast cancer, highlight the growing arsenal of Breast Cancer Approved Drugs.
The emerging pipeline for HR+/HER2− breast cancer is crowded with potential therapies targeting various mechanisms. Novel CDK4/6 inhibitors, oral SERDs, AKT inhibitors, mTOR inhibitors, SERMs, PI3K inhibitors, and TROP-2 targeting antibody-drug conjugates (ADCs) are all showing promise. Drugs like elacestrant (ORSERDU), an oral SERD approved in January 2023, and TRUQAP (capivasertib), approved in November 2023, are reshaping the treatment landscape.
The evolving landscape of HR+/HER2− breast cancer treatment holds the potential for more tailored and effective treatments, providing renewed hope for patients facing this challenging disease. The continued research into factors such as mutations, biomarker discovery, and improved therapeutic approaches is essential for advancing breast cancer care. The dedication of physicians, oncology professionals, and the entire healthcare community to this cause is evident in the transformative changes occurring in the treatment of metastatic HR+/HER2− breast cancer.
Conclusion
In conclusion, the treatment and management of HR+/HER2− breast cancer are advancing at an unprecedented pace, driven by innovative therapies and a deeper understanding of the disease. The focus on new Breast Cancer Approved Drugs and targeted therapies is reshaping the landscape, offering hope and improved outcomes for patients worldwide. As we move forward, the integration of these novel treatments will continue to transform the standards of care, ensuring that patients receive the most effective and personalized treatment options available.

Downloads
Article in PDF
Recent Articles
- Will Merck’s P2X3 Receptor Antagonist for Chronic Refractory Cough Treatment Be Able to Turn FDA’...
- Antibody-drug Conjugates in Oncology: An Overview of the Current and Future Treatment Landscape
- Cardiovascular Disease: A major cause of health loss and a global burden
- Noxxon’s NOX-A12 clinical trial; Exact Sciences buys PreventionGenetics; Nuvalent’s clinical trai...
- Liquid Biopsy: Revolutionary Technique for Cancer Diagnosis