Multiple MYELOMA drugs Market.
Sep 23, 2025
Merck Wins FDA Approval for KEYTRUDA QLEX for Subcutaneous Use in Adults With Solid Tumors; Incyte Gains FDA Nod for OPZELURA Cream in Children Aged 2–11 With Atopic Dermatitis; Minovia Therapeutics Receives FDA Fast Track Designation for MNV-201 in Myelodysplastic Syndrome; MavriX Bio Secures FDA Fast Track for MVX-220 Gene Therapy in Angelman Syndrome; Biocon Biologics Gets FDA Approval for Denosumab Biosimilars BOSAYA and AUKELSO
FDA Approves Merck’s KEYTRUDA QLEX for Subcutaneous Use Across Multiple Solid Tumors Merck announced that the FDA has approved KEYTRUDA QLEX (pembrolizumab and berahyaluronidase alfa-pmph) for subcutaneous administration in adults across most of KEYTRUDA’s approved solid tumor indications. Unlike the intravenous...
Read More...
May 06, 2025
Abeona Secures FDA Nod for ZEVASKYN, the First Gene Therapy for RDEB; AbbVie Gains FDA Approval for RINVOQ in Giant Cell Arteritis; Lantern Pharma Advances LP-184 with IND Clearance for TNBC Trial; Ichnos Glenmark Earns FDA Fast Track for ISB 2001 in Multiple Myeloma; Rezolute Wins Breakthrough Therapy Designation for Ersodetug in Tumor-Induced Hypoglycemia
FDA Approves Abeona’s ZEVASKYN as First Cell-Based Gene Therapy for RDEB The FDA approved Abeona Therapeutics’ ZEVASKYN (prademagene zamikeracel, or pz-cel), the first and only autologous cell-based gene therapy for treating wounds in patients with recessive dystrophic epidermolysis bullosa (RDEB). ZEVASKYN is d...
Read More...
Apr 02, 2025
Revolutionary Advances and Bright New Horizons in Multiple Myeloma Treatment
Multiple myeloma, a complex blood cancer rooted in the bone marrow, affects 160K people globally each year, with a mortality rate of 106K. According to DelveInsight, nearly 75K new cases were reported across seven major markets in 2023, a figure expected to climb in the coming years. Yet, amid these sobering statis...
Read More...
Mar 14, 2025
5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
The success of CAR-T therapies like Bristol-Myers Squibb/Bluebird bio’s ABECMA and Janssen’s CARVYKTI is reshaping treatment paradigms, challenging traditional options like stem cell transplants and proteasome inhibitors. The multiple myeloma market size in the US is responding rapidly from ~USD 15 billion, with in...
Read More...
Aug 05, 2024
DARZALEX FASPRO Regimen Approval: Johnson & Johnson Howling Success in Multiple Myeloma
Despite significant progress in multiple myeloma treatment with a range of biological drugs and their combinations—such as IMiDs (BMS REVLIMID, POMALYST/IMNOVID), anti-CD38 monoclonal antibody drugs (DARZALEX, SARCLISA), the anti-SLAM7 monoclonal antibody (AbbVie and BMS multiple myeloma drug EMPLICITI), and new pr...
Read More...
Jul 30, 2024
The Changing Landscape of Multiple Myeloma Therapies Market
Multiple Myeloma, the second most common type of blood cancer after non-Hodgkin lymphoma, is characterized by the presence of malignant plasma cells in the bone marrow. The diagnosis involves identifying these highly abnormal and unstable cells, which proliferate aggressively and spread through the bloodstream and ...
Read More...
Jul 25, 2024
CELMoDs – A Worthy Successor to REVLIMID?
Over the years, REVLIMID has become an integral weapon in the cancer therapeutics armory. This odyssey of REVLIMID in the multiple myeloma segment began in 2005, when it was approved by the US FDA for the treatment of adult patients with multiple myeloma, in combination with dexamethasone. As the wise say,...
Read More...
Jul 24, 2024
Transforming Multiple Myeloma Treatment: The Promise of Novel Drug Classes
Multiple myeloma is the second most prevalent blood cancer diagnosis in the United States, after non-Hodgkin lymphoma. Males are significantly more likely than females to have multiple myeloma. Myeloma incidence is strongly correlated with age, with the highest rates of diagnosis occurring in adults aged 65 years a...
Read More...
Mar 29, 2019
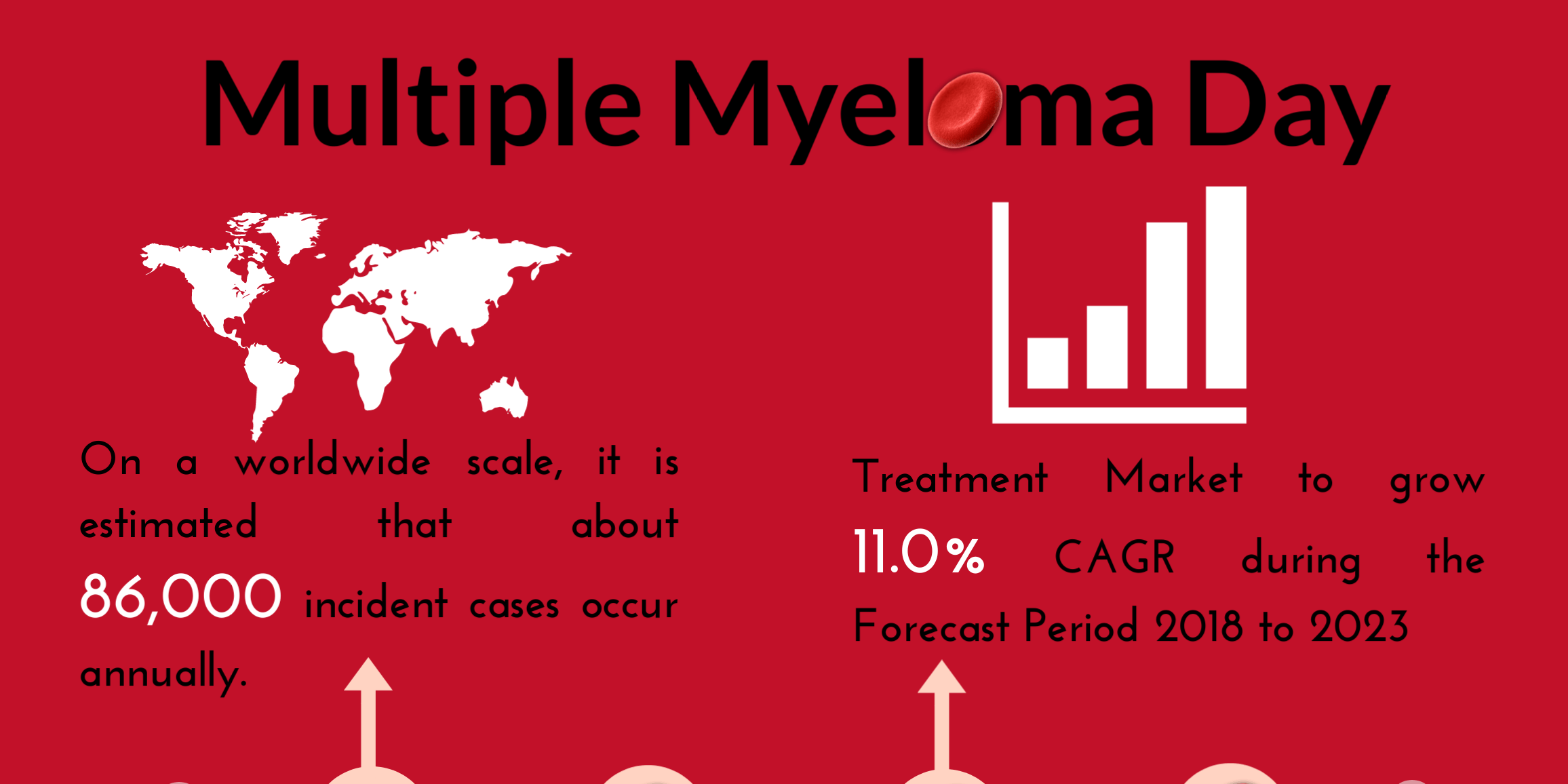
Multiple Myeloma Day
Multiple Myeloma Day Multiple Myeloma is relatively a rare disease. It accounts for approximately 1% of all cancers and 10% of all blood and bone marrow cancers. For more information on Multiple Myeloma.
Read More...



-Agonist.png)

