Pharmaceutical Industry
Jan 06, 2025
Parkinson’s and Tuberculosis Share a Common Protein That Could Provide Better Drugs for Both
Parkinson's disease and tuberculosis are two significant health challenges that affect millions worldwide. Recent research has unveiled a fascinating connection between these two diseases through a common protein known as Parkin. Understanding this link not only sheds light on the mechanisms of both diseases but al...
Read More...
Sep 04, 2024
Nonalcoholic Steatohepatitis (NASH): A Growing Epidemic
Obesity and diabetes are a global epidemic contributing to an increasing prevalence of related systemic disorders, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Nonalcoholic Steatohepatitis is a subtype of NAFLD (Nonalcoholic Fatty Liver disease) that is characterized...
Read More...
Sep 04, 2024
Meeting the Unmet: Nonalcoholic Steatohepatitis (NASH)
Nonalcoholic Steatohepatitis (NASH) is a severe form of nonalcoholic fatty liver disease (NAFLD), characterized by liver inflammation and damage due to fat accumulation. Unlike simple fatty liver, NASH can progress to serious conditions such as fibrosis, cirrhosis, and liver failure. This chronic liver disease is i...
Read More...
Dec 09, 2022
ASH 2022 Annual Meeting – Insights Into Major Diffuse Large B-cell Lymphoma (DLBCL) Abstract and Breakthroughs
Blood cancers have seen tremendous advancements in the past few years owing to the dedication of the researchers who are putting their endless efforts just to bring innovation into the lives of suffering patients. These experiments and their upshots are always brought to us through various platforms, the American S...
Read More...
Jun 22, 2022
How is Nanomedicine Transforming the Dynamics of the Healthcare Industry?
Over the span of the last decade, new and revolutionary technology has been introduced into our daily lives - nanotechnology. It has been applied in multiple fields through an integrated approach. With a rise in the number of applications and products containing nanomaterials, nanotechnology now is a very integral ...
Read More...
May 02, 2022
Commercial Roadmap for the Launch: What can Biopharmaceuticals do to Increase the Odds of Success?
The Pharma and Biotech Healthcare market has been an ever-changing industry in terms of the type of biopharmaceutical products innovated and launched, evolving from management of chronic diseases in the 1990s to the expansive marketing of generic drugs, to now moving onto personalized therapies. To increase the suc...
Read More...
Feb 11, 2022
Mapping the Biggest Pharmaceutical Companies by Continents
Today, the most important factor affecting the world’s economy is the Global Pharmaceutical Industry. This industry is at the forefront driving about one trillion dollars annually. Pharmaceutical companies are driving a major share of the global economy with the rapidly advancing technological and scientific base, ...
Read More...
Oct 27, 2021
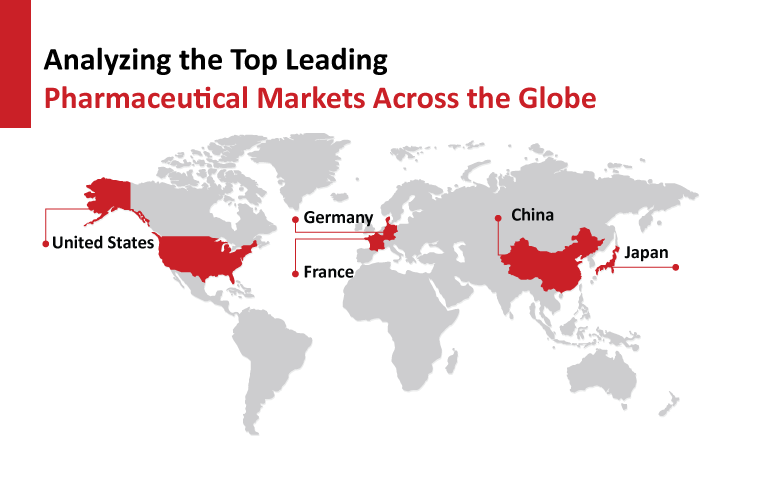
Which Countries Top the Chart in Global Pharmaceutical Market?
The Pharmaceuticals segment of the Healthcare Industry has a significant market across the globe, which has grown quite extensively in the past few decades. The pharmaceutical sector has always been at the forefront of drug discovery, development, production, and marketing. The pharmaceutical industry in the twenty...
Read More...
Jul 10, 2018
Notizia
Arming antibiotics with a vaccination-like immune response Harnessing the ability of the body’s immune system has already well-tried to be effective in treating cancer. Marcos Pires, Ph.D., a Lehigh associate professor of biochemistry performed a test on it. To make it worse, these microorganism perpetually evolv...
Read More...







-Agonist.png)

