Abbvie-Genmab’s EPKINLY/TEPKINLY Performance in DLBCL Treatment: The First CD20XCD3 Bispecific Antibody
Jun 10, 2025
Table of Contents
A new era in lymphoma therapy began in 2023 when AbbVie and Genmab’s EPKINLY/TEPKINLY became the first CD20xCD3 bispecific antibody approved in the US for the treatment of patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). This groundbreaking approval marked a major milestone in immunotherapy, offering a novel, off-the-shelf alternative to CAR-T therapy. Just a month later, Roche’s COLUMVI entered the scene, intensifying the competition. Unlike CAR-T therapies, both EPKINLY and COLUMVI are fixed-dose, subcutaneous treatments designed to be more accessible and scalable, poised to serve a broader patient population in the R/R DLBCL space. As the treatment landscape evolves rapidly, these bispecific antibodies are setting new standards in efficacy, convenience, and patient reach.
The FDA granted accelerated approval to EPKINLY in May 2023 based on its significant effects on response rate and the lasting nature of the response in DLBCL patients. The approval was supported by results from the LBCL group of the Phase II pivotal EPCORE NHL-1 (NCT03625037) trial, an open-label, multicenter study. Even in the extended 2.5-year follow-up trial of the EPCORE NHL-1 trial, EPKINLY demonstrated deep and durable responses and long-term efficacy in LBCL patients. These findings demonstrated that subcutaneous EPKINLY resulted in profound and long-lasting responses in these patients. The ORR was 61%, with 38% of patients achieving complete responses. Among those who responded to treatment, the median follow-up period was 9.8 months, and the estimated median duration of response (DOR) was 15.6 months.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Will Roche’s Crovalimab An Answer to AstraZeneca PNH Treatment Drugs?
- Boehringer Ingelheim; Roche demonstrated new analyses of their drugs in patients with ILDs during...
- AbbVie, Sofinnova back; Gilead, Kite ink; Biogen aims filing; Reversing diabetes
- Most Promising Oncological Drugs Expected to Launch in 2022
- Thumbs down to NKTR-181, Lilly expands its insulin armamentarium
In September 2023, the European Commission also granted conditional marketing authorization to TEPKINLY (marketed as EPKINLY in the US and Japan) as a monotherapy for the treatment of adult patients with R/R DLBCL following two or more prior lines of systemic therapy. Additionally, it also received approval from the Japan MHLW in September 2023 for R/R LBCL.
EPKINLY Primed to Lead in the CD20xCD3 Drug Class
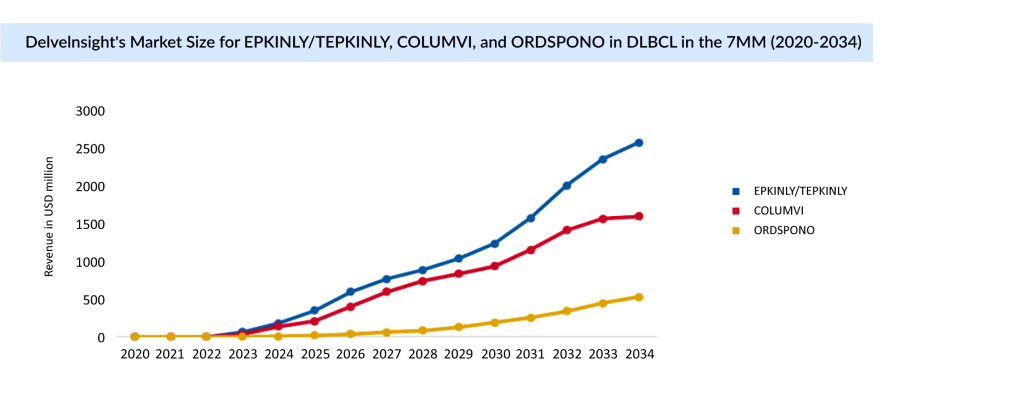
Since 2023, EPKINLY has received regulatory approvals in more than 50 countries. It is the first bispecific approved in the world with a dual indication in 3L+ R/R DLBCL. EPKINLY’s strong launch in R/R DLBCL and follicular lymphoma continues to gain traction, generating USD 281 million in 2024.
One of the notable advantages of EPKINLY is its suitability for administration in community settings, as it does not require specialized registered centers like chimeric antigen receptor (CAR) T-cell therapy does. Consequently, more physicians can now have access to this drug, potentially leading to its inclusion in the treatment plans for a larger number of patients.
EPKINLY holds an additional advantage with its subcutaneous formulation, offering a more convenient administration method compared to COLUMVI’s intravenous infusion. This convenience factor becomes even more significant when considering future combinations and treatment approaches.
Recent Developments
At the American Society of Hematology (ASH) 2024 held in December, AbbVie presented promising results from the EPCORE NHL-1 and NHL-2 trials of EPKINLY. In EPCORE NHL-2, 91% of patients (n = 33) achieved minimal residual disease (MRD) negativity, with manageable safety signals—most common adverse events included neutropenia (70%), anemia (69%), and cytokine release syndrome (CRS, 60%). CRS events were mostly low-grade and resolved without discontinuation.
In EPCORE NHL-1, three-year follow-up data from 157 patients with Relapsed/Refractory LBCL showed durable efficacy, with an overall response rate of 59%, complete response rate of 41%, and a median duration of CR of 36.1 months. MRD negativity was achieved in 45.4% (n = 119), reinforcing epcoritamab’s potential in difficult-to-treat cases. It is worth noting that another data readout of EPKINLY for first-line DLBCL (EPCORE DLBCL-2) and second-line and above DLBCL (EPCORE DLBCL-1) transplant ineligible is anticipated by the end of 2026.
Since EPKINLY is currently approved for third-line and beyond DLBCL patients, Genmab anticipates expanding its use to second-line R/R DLBCL between 2027 and 2030, and to first-line DLBCL between 2027 and 2031.
COLUMVI and ORDSPONO, the Closest Competitors to EPKINLY for DLBCL Treatment
COLUMVI has emerged as a strong contender against EPKINLY in the field of DLBCL treatment, offering a promising option for people with relapsed or refractory (R/R) DLBCL. In June 2023, Roche secured FDA approval for COLUMVI for adults with R/R DLBCL and large B-cell lymphoma (LBCL) after at least two prior lines of therapy. This accelerated approval was based on response rates and durability of response from the Phase I/II NP30179 study, positioning COLUMVI as a fixed-duration treatment that allows potential time off therapy.
The momentum continued in July 2023, when the European Commission (EC) granted conditional marketing authorization for COLUMVI , making it the first CD20xCD3 T-cell-engaging bispecific antibody available in Europe for DLBCL treatment. This approval reinforced its role as a groundbreaking therapy for people with R/R DLBCL, further intensifying the competition between COLUMVI and EPKINLY in the evolving treatment landscape.
ORDSPONO, another bispecific antibody has turned out to be a market contender for EPKINLY, it received approval from the EC in August 2024 for R/R DLBCL after two or more lines of systemic therapy. Further with Fast Track Designation (FTD), pivotal Phase II trials (NCT03888105) is ongoing for patients with R/R DLBCL and follicular lymphoma.
Earlier in December 2020, the FDA placed a partial hold on Regeneron’s bispecific antibody ORDSPONO due to concerns about cytokine release syndrome in the Phase I ELM-1 study. To lessen this side effect, a longer step-up dosing regimen was implemented and the hold was lifted in May 2021.
Emerging Bispecific Antibodies for People With DLBCL
Several other CD20xCD3 bispecific antibodies are under active study and have demonstrated promising activity in DLBCL, including LUNSUMIO (mosunetuzumab) and AZD0486.
Roche, in collaboration with Biogen, has developed the CD20xCD3 bispecific antibody LUNSUMIO (mosunetuzumab). The company is currently evaluating LUNSUMIO in combination with POLIVY in a Phase III trial (SUNMO) for second-line diffuse large B-cell lymphoma (2L DLBCL). This trial is primarily being conducted in the US and Japan, with additional sites outside the seven major markets (7MM). A pivotal Phase III readout for LUNSUMIO in 2L+ DLBCL is expected in 2025, with regulatory submissions planned in both the US and Japan within the same year. Despite this
LUNSUMIO is also a fixed-duration treatment that can be administered in the outpatient setting, which could allow people the possibility of experiencing a lasting remission with a treatment-free period.

Another asset, AZD0486, being developed by AstraZeneca, is being studied as a monotherapy for relapsed or refractory B-cell R/R DLBCL. Early trial results show high complete remission rates, manageable side effects, and durable responses. Notably, severe Cytokine Release Syndrome (CRS) has not been observed, with mild to moderate neurological events in 24% of patients, and only one severe case. AstraZeneca anticipates data from the Phase I/II SOUNDTRACK-E (NCT06564038) and Phase I trial (NCT04594642) beyond 2025. Looking ahead, the company plans to initiate Phase III trials for first-line DLBCL post-2030.
Conclusion, Challenges, and Future Directions in the Evolving Landscape of DLBCL Treatment
COLUMVI has rapidly positioned itself as a strong contender in the competitive DLBCL treatment space, offering a fixed-duration, off-therapy option for Relapsed/Refractory patients. With regulatory approvals in both the US and Europe, it has carved a niche against rivals like EPKINLY and ORDSPONO. As the bispecific antibody landscape evolves, Roche’s broader pipeline, including LUNSUMIO, along with emerging therapies from AstraZeneca, signals an intensifying race to redefine DLBCL care through innovative, targeted, and patient-friendly treatments.
The field of bispecific antibodies is still in its early stages but holds immense promise in harnessing the power of the immune system to improve outcomes for patients with DLBCL. For relapsed or refractory DLBCL, these therapies offer a potentially more effective and accessible treatment option compared to prior standards.
Despite the promising clinical progress of CD20xCD3 bispecific antibodies in diffuse large B-cell lymphoma (DLBCL), several critical challenges must still be addressed to fully unlock their therapeutic potential. Key among these are optimizing dosing strategies, minimizing adverse effects, particularly cytokine release syndrome, and determining the most effective sequencing with other therapies. One of the most pressing clinical questions is whether bispecific antibodies should be administered before or after CAR-T cell therapy, and whether they can be safely and effectively combined with chemotherapy in the frontline setting. These questions remain open, with ongoing trials actively working to define their answers.
Currently, bispecific antibodies are carving out a niche in the third-line and beyond treatment setting for DLBCL. However, with encouraging efficacy and manageable safety profiles, they are poised to move earlier in the treatment paradigm. Long-term follow-up data and ongoing clinical research could soon establish these agents as a new cornerstone in DLBCL management, especially for patients who are ineligible for or relapse after CAR-T therapy, or in settings where CAR-T is not readily accessible due to logistical or financial barriers.
Importantly, emerging evidence indicates that prior CAR-T therapy does not significantly reduce response rates to CD3/CD20 bispecific antibodies. However, the impact of prior bispecific antibody use on subsequent CAR-T therapy remains uncertain. As CAR-T therapies continue to demonstrate curative potential and are increasingly adopted as second-line options, bispecifics are likely to serve as vital alternatives or complements, particularly as bridging therapies or in patients who cannot receive cell-based treatments.
Looking ahead, bispecific antibodies have the potential to be integrated into first-line regimens for high-risk patients and combined with chemoimmunotherapy to enhance frontline outcomes. As their role continues to expand, CD3/CD20 bispecifics are expected to reshape the therapeutic landscape of DLBCL by offering greater flexibility, accessibility, and efficacy across multiple lines of therapy.

Downloads
Article in PDF
Recent Articles
- Prometic bags $50M; Vedanta receives $12M; Humira biosimilar deal
- Dubai: A leading and exciting pharma hotspot
- ASH 2022 Annual Meeting – Insights Into Major Diffuse Large B-cell Lymphoma (DLBCL) Abstrac...
- Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabha...
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan