7 Emerging Spinal Muscular Atrophy Therapies Offering Hope for Patients
May 19, 2025
The approval of three spinal muscular atrophy therapies—SPINRAZA (Ionis/Biogen), EVRYSDI (Roche), and the groundbreaking gene therapy ZOLGENSMA (Novartis) has significantly advanced treatment options for a condition that previously had limited solutions. These spinal muscular atrophy (SMA) therapies offer patients more personalized care.
Nonetheless, managing SMA effectively requires more than just medications; it calls for a comprehensive, multidisciplinary approach that incorporates supportive care to reduce complications and improve the quality of life for patients and their families. This integrative model relies on coordinated efforts among neurologists, physical therapists, nutritionists, and other specialists to foster better long-term health outcomes.
Multiple companies are actively working to enhance SMA treatments. Leading candidates in the development spinal muscular atrophy pipeline include Scholar Rock’s Apitegromab, Novartis’ intrathecal version of ZOLGENSMA (OAV101 IT), Biohaven’s Taldefgrobep Alfa, Biogen’s high-dose SPINRAZA and BIIB115, Chugai/Roche’s GYM329/RG6237, NMD Pharma’s NMD670, and several others.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Spinal Muscular Atrophy Market
- How Spinal Muscular Atrophy Therapies transform market?
- Spinal Muscular Atrophy Market is expected to augment at a CAGR of 10.42%
- Novartis Secures FDA Approval for ITVISMA; Kelun-Biotech’s Phase III Study Shows Sac-TMT + Keytru...
- SpringWorks’s Desmoid Tumors Therapeutic, Nirogacestat; Orphan Drug Designation to AskBio’s AB-10...
Here’s an in-depth look at seven promising emerging therapies that could transform the future of spinal muscular atrophy treatment.
Scholar Rock’s Apitegromab
Apitegromab (SRK-015), developed by Scholar Rock, is an investigational therapy for spinal muscular atrophy that works by selectively inhibiting the activation of latent (inactive) myostatin, rather than targeting the already-active form or its receptor. This unique mechanism aims to prevent myostatin activation within muscle tissue.
The drug has received multiple regulatory designations: Orphan Drug, Fast Track, and Rare Pediatric Disease designations from the US FDA, as well as Orphan Medicinal Product status from the European Commission. Additionally, the European Medicines Agency (EMA) has granted it Priority Medicines (PRIME) designation for the treatment of SMA.
In March 2025, Scholar Rock announced that the FDA had accepted its Biologics License Application (BLA) for apitegromab. The application will be reviewed under the FDA’s priority review pathway, with a Prescription Drug User Fee Act (PDUFA) target decision date set for September 22, 2025. The priority review status indicates that the FDA believes apitegromab has the potential to significantly improve the treatment of spinal muscular atrophy.
Additionally, the company has submitted a Marketing Authorisation Application (MAA) to the European Medicines Agency (EMA) for the use of apitegromab in treating SMA. The EMA has validated the submission, confirming it is complete and ready for formal review.
These regulatory filings are supported by positive efficacy and safety results from the pivotal Phase III SAPPHIRE trial (NCT05156320), with topline data released in October 2024, along with data from the earlier Phase II TOPAZ trial and the long-term ONYX extension study. Findings from the SAPPHIRE trial, including both primary and secondary endpoints, were recently presented at the 2025 Muscular Dystrophy Association (MDA) Clinical & Scientific Conference.
SAPPHIRE met its primary endpoint, showing a statistically significant and clinically meaningful improvement in motor function in SMA patients treated with apitegromab alongside ongoing SMN-targeted therapies (nusinersen or risdiplam), compared to those receiving placebo with the same standard treatments. Motor function was assessed using the Hammersmith Functional Motor Scale Expanded (HFMSE), the recognized gold standard.
In preparation for potential approvals, Scholar Rock is gearing up for a commercial launch in the US in 2025, followed by a European launch in 2026.
Novartis’ ZOLGENSMA
Novartis is advancing efforts to broaden the use of ZOLGENSMA to treat older children with spinal muscular atrophy. In March 2025, the company announced its intention to pursue regulatory approval in the first half of the year for a new formulation of the gene therapy that is administered via spinal injection, rather than the existing intravenous (IV) infusion. The company first shared top-line results from its Phase III STEER trial in December and later presented comprehensive data at the Muscular Dystrophy Association (MDA) Clinical and Scientific Conference.
The STEER study evaluated the intrathecal version of the therapy, known as OAV101 IT, in 126 treatment-naïve patients aged 2 to 17 with SMA type 2 who could sit but had never walked independently. The trial achieved its primary endpoint, showing a statistically significant improvement of 2.39 points on the Hammersmith Functional Motor Scale – Expanded (HFMSE), compared to a 0.51-point improvement in the placebo group.
Additionally, Novartis conducted the Phase IIIb STRENGTH trial in 27 children aged 2 to 17 who had previously received other SMA treatments, including Biogen’s SPINRAZA and Roche’s EVRYSDI, but had discontinued them. The findings indicated that OAV101 IT helped maintain motor function, with a 1.05-point improvement in HFMSE scores over one year. These patients had typically been on prior treatments for three to four years.
In the STEER trial, the most common serious side effects in the OAV101 IT group were pneumonia and vomiting. Liver enzyme elevations occurred infrequently, were mostly mild and transient, and no cases met Hy’s law criteria. There were two reported cases of peripheral sensory neuropathy, although the data remain blinded, so it’s unclear which treatment group those patients were in. Symptoms were addressed with additional treatments and improved over time.
In the STRENGTH trial, about 48% of participants experienced treatment-related adverse events. Hepatotoxicity occurred in four patients (14.8%), but no deaths or significant safety issues led to study discontinuation.
The FDA had previously halted testing of the therapy in 2019 due to concerns about inflammation in the dorsal root ganglia seen in animal studies. In STEER, signs of this issue appeared in 2.7% of patients in the treatment group and 2% in the control group. In STRENGTH, the rate was 7.4%. These events were considered mild and resolved without intervention.
Novartis views OAV101 IT as a vital step toward ensuring access to gene therapy for all SMA patients, not just infants. Currently, ZOLGENSMA is approved only for children under two with bi-allelic mutations in the SMN1 gene. The new intrathecal version uses a fixed dose rather than a weight-based one, potentially making it a safer and more practical option for older, heavier patients.
The company has yet to reveal the anticipated price of OAV101 IT if approved, stating that pricing will be based on the therapy’s overall value to patients and the healthcare system. The current version of ZOLGENSMA costs over $2 million.
Biohaven’s Taldefgrobep Alfa
Taldefgrobep is an experimental, muscle-targeted recombinant protein designed to increase muscle mass and strength in individuals with spinal muscular atrophy, particularly when used alongside existing approved treatments. It works by targeting myostatin, a protein that naturally restricts skeletal muscle growth, through two pathways: directly reducing myostatin levels and inhibiting downstream signaling. Blocking myostatin is considered a promising therapeutic approach for both children and adults with neuromuscular diseases, as elevated myostatin can hinder the muscle growth needed for developmental and functional progress.
In November 2024, Biohaven shared updates on taldefgrobep alfa’s development in both SMA and obesity. In the RESILIENT SMA trial, the drug showed clinically meaningful improvements in motor function across all timepoints using the Motor Function Measurement-32 (MFM-32) scale. However, it did not achieve statistical significance in the primary endpoint at Week 48 compared to the placebo plus standard of care (SOC).
Encouraging efficacy signals were seen in several clinically and biologically defined subgroups, including variations in age, ability to walk, background therapy, and baseline myostatin levels. Among the largest demographic group (87% Caucasian, n=180), statistically significant improvements were seen on the MFM-32 scale at all timepoints, including Week 48 (p < 0.05). Additionally, a subgroup of patients with detectable baseline myostatin (n=123) demonstrated stronger efficacy results (p = 0.02).
A meeting with the FDA to review the registrational strategy for SMA is scheduled for the first half of 2025. Additionally, Biohaven plans to initiate taldefgrobep Phase II study in obesity in 1H 2025.
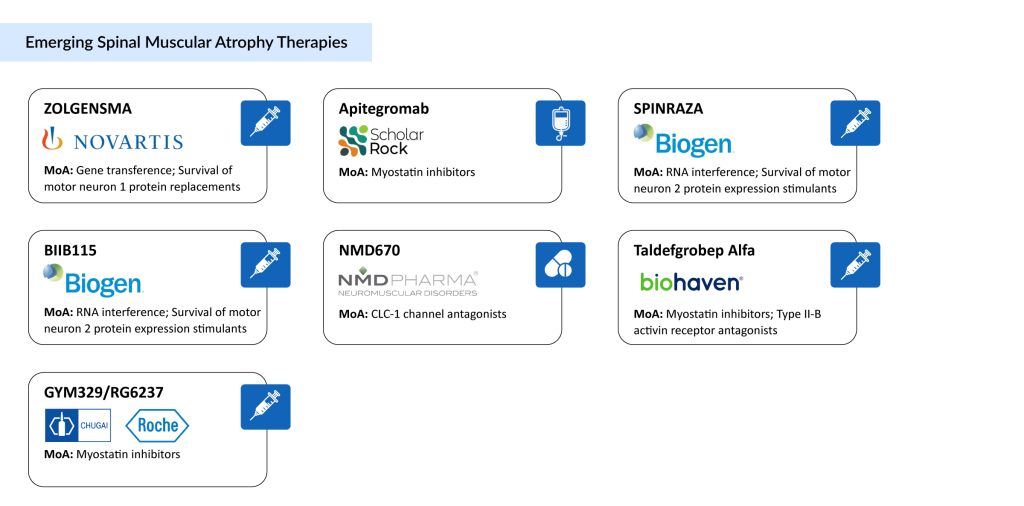
Biogen’s SPINRAZA and BIIB115
SPINRAZA has received approval in over 71 countries for the treatment of spinal muscular atrophy in infants, children, and adults. As a cornerstone therapy for SMA, it has been administered to more than 14,000 individuals globally. The approved 12 mg dosing schedule includes four loading doses over about 60 days, followed by maintenance doses every four months.
SPINRAZA is an antisense oligonucleotide (ASO) designed to address the root cause of motor neuron degeneration in SMA by consistently boosting the production of full-length survival motor neuron (SMN) protein. The drug is delivered directly into the central nervous system, where motor neurons are located, to target the disease at its origin.
The treatment has demonstrated long-term efficacy across various ages and types of SMA, with a well-established safety record supported by up to 10 years of patient data and extensive real-world use. The SPINRAZA development program includes over 10 clinical trials involving more than 460 participants, including two pivotal randomized studies (ENDEAR and CHERISH). The most frequently reported side effects in trials included respiratory infections, fever, constipation, headaches, vomiting, and back pain. Routine lab tests are used to monitor for potential risks such as kidney toxicity and coagulation issues, including severe thrombocytopenia observed with some ASOs.
Biogen holds the global rights to develop, produce, and commercialize SPINRAZA through a license from Ionis Pharmaceuticals, Inc. In January 2025, Biogen announced that the FDA had accepted its supplemental New Drug Application (sNDA), and the EMA had validated a submission for a higher-dose regimen of nusinersen. This proposed regimen features a faster initial dosing phase, two 50 mg doses 14 days apart, and higher maintenance doses of 28 mg every four months, compared to the current standard.
Despite SPINRAZA’s success, Biogen is pursuing further innovation. In January 2022, the company exercised its option to license BIIB115, another ASO developed by Ionis that may allow for longer intervals between doses.
BIIB115 is currently being evaluated in a Phase I clinical trial to assess its safety and pharmacokinetics in healthy adult males and pediatric SMA patients previously treated with ZOLGENSMA. Initial results from this study are anticipated in November 2026.
Chugai/Roche’s GYM329/RG6237
GYM329, developed by Chugai, is a next-generation antibody that leverages the company’s proprietary antibody engineering technologies, such as the recycling antibody and sweeping antibody platforms. Latent myostatin, primarily secreted by muscle cells in an inactive form, becomes active through enzymes like BMP-1 that degrade proteins. Once activated, myostatin suppresses muscle growth and hypertrophy. By blocking myostatin’s activity, GYM329 is designed to counteract muscle atrophy and improve muscle strength.
A global Phase II/III spinal muscular atrophy clinical trial is currently assessing the safety and effectiveness of GYM329 (RO7204239), an experimental anti-myostatin antibody aimed at promoting muscle growth, in combination with EVRYSDI for the treatment of SMA.
NMD Pharma’s NMD670
NMD670 is the lead development candidate from NMD Pharma. It is a first-in-class small molecule that targets and inhibits the skeletal muscle-specific chloride ion channel 1 (CIC-1). NMD Pharma has shown that inhibiting CIC-1 enhances the muscle’s sensitivity to weak nerve signals, thereby improving neuromuscular communication and restoring muscle function. This innovative approach has demonstrated clinical proof-of-concept in myasthenia gravis and preclinical potential in spinal muscular atrophy, Charcot-Marie-Tooth disease, and sarcopenia.
NMD670 is currently being evaluated in a Phase II clinical trial for SMA. The study, which began dosing patients in late 2023, is assessing improvements in a six-minute walk test in ambulatory adults aged 18 to 75. The spinal muscular atrophy clinical trial is expected to conclude in January 2026.
The introduction of new spinal muscular atrophy therapies has the potential to gradually influence the rare disease treatment landscape. As newborn screening becomes more widespread and awareness of the condition grows, the eligible patient population may broaden. At the same time, a more competitive market could influence pricing trends, reimbursement discussions, and access strategies. These developments may also encourage continued innovation in the broader fields of neuromuscular and genetic disorders. Over time, such spinal muscular atrophy therapies could contribute to evolving standards of care and shifting expectations around the long-term management of SMA.

Downloads
Article in PDF
Recent Articles
- Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
- Top 12 Most Expensive Drugs in the US Healthcare Market
- FDA’s Ok to Roche’s Oral SMA Therapy; Roche’s Etrolizumab Mixed Results in Ulce...
- Spinal Muscular Atrophy Emerging therapies
- Spinal Muscular Atrophy: Current and Emerging Therapies