Atherosclerotic Cardiovascular Disease (ASCVD) Treatment Approaches: Beyond Statins
Jan 29, 2024
Table of Contents
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and mortality worldwide, encompassing conditions such as coronary artery disease and stroke. The growing occurrence of ASCVD, fueled by elevated risk factors, cardiovascular disease, and diabetes, is contributing to an expanding market for pharmaceuticals, medical devices, and diagnostic tools, as it impacts a substantial segment of the population.
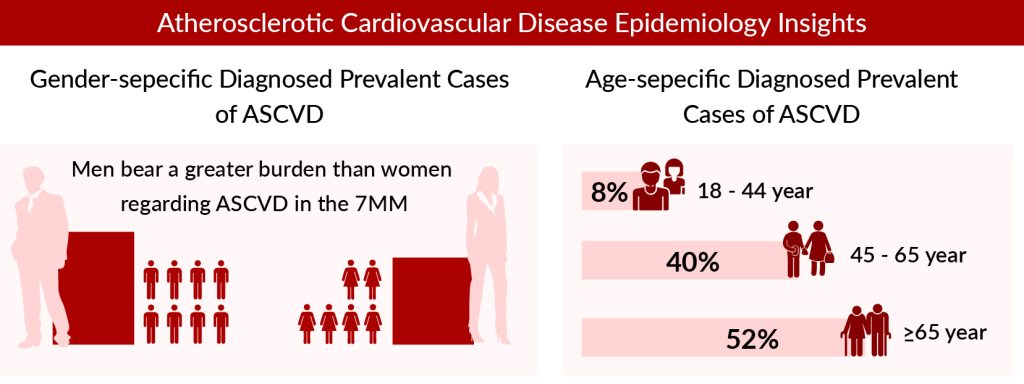
According to our analysis, the total diagnosed prevalent cases of ASCVD in the 7MM were 55 million cases in 2022 and this number is predicted to climb at a substantial CAGR throughout the research period (2019–2032). DelveInsight’s analyst has classified ASCVD cases with comorbidities into distinct categories, including recent acute coronary syndrome (ACS), ischemic stroke, peripheral arterial disease (PAD), and other coronary heart disease (CHD) conditions, and other comorbidities. Other comorbidities in ASCVD patients include the cases of Chronic Kidney Disease (CKD). Other CHD conditions accounted for the highest cases among Comorbidity-specific Diagnosed Prevalent Cases of ASCVD.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- PCSK9 Inhibition in Cardiovascular Care: Unmet Needs, Market Evolution, and Emerging Players
- PCSK9 Inhibitors: A New Era for Cholesterol Management
- Rejections and Withdrawals: Familial Chylomicronemia Syndrome Market Needs a Therapy sufficiently...
- Amylyx’s AMX0114 Fast Tracked by FDA for ALS; Cellectar’s Iopofosine I 131 Granted FDA Breakthrou...
- Arrowhead Hits the Mark in FCS Treatment with REDEMPLO Approval—Now Comes the Ionis Challenge
The management of ASCVD includes the use of medications and invasive interventions such as PCI and CABG. Statins are crucial for preventing ASCVD and are considered a cornerstone in ASCVD treatment. Due to the potential risks linked to statins, there are various alternative treatment choices, including ezetimibe, PCSK9 inhibitors, omega-3 fatty acids, and apolipoprotein(A) inhibitors.
Statins: The Cornerstone of ASCVD Treatment
Statins, a class of drugs designed to lower cholesterol levels in the bloodstream, have emerged as the cornerstone of ASCVD treatment. Statins work by inhibiting the enzyme HMG-CoA reductase, a key player in the cholesterol synthesis pathway, leading to a reduction in low-density lipoprotein (LDL) cholesterol levels. Extensive clinical trials have consistently demonstrated the efficacy of statins in lowering the risk of cardiovascular events and improving overall outcomes in patients with or at risk for ASCVD. Beyond their lipid-lowering effects, statins exhibit anti-inflammatory and plaque-stabilizing properties, contributing to their comprehensive cardiovascular benefits. As a result, these medications are recommended as the first-line therapy for managing lipid levels in individuals with ASCVD or those at high risk. While other interventions and medications may complement statin therapy in specific cases, their pivotal role in reducing cardiovascular morbidity and mortality solidifies statins as the cornerstone of ASCVD treatment strategies.
Other ASCVD Treatment Options Apart From Statins
In addition to statins, a varied range of drug categories is utilized in handling ASCVD. These include antiplatelet medications such as aspirin to prevent blood clotting, beta-blockers to alleviate heart strain, ACE inhibitors/ARBs to regulate blood pressure, diuretics to manage fluid balance, nitrates to widen vessels, anticoagulants to prevent clots, ezetimibe to inhibit cholesterol absorption, PCSK9 inhibitors for additional reduction in LDL cholesterol, and diabetes drugs to assist in controlling blood sugar. Together, these medications address key factors contributing to ASCVD risk and support overall cardiovascular well-being.
Numerous non-generic medications such as LEQVIO (inclisiran), NEXLETOL/NILEMDO (bempedoic acid), NEXLIZET/NUSTENDI (bempedoic acid and ezetimibe), PRALUENT (alirocumab), XARELTO (rivaroxaban), and REPATHA (evolocumab) are presently accessible in the market for ASCVD treatment.
LEQVIO (inclisiran) received FDA approval in 2021 for treating ASCVD. As a member of the PCSK9 inhibitor category, its effectiveness in reducing LDL cholesterol shows promise for individuals with familial hypercholesterolemia or ASCVD who haven’t reached their target LDL cholesterol levels with alternative lipid-lowering treatments. However, it has not yet gained approval in Japan. ACL inhibitors, a groundbreaking drug class, act on the enzyme ACL to impede cholesterol production in the liver by obstructing the HMG-CoA enzyme. In 2020, the FDA sanctioned NEXLETOL/NILEMDO (bempedoic acid) and NEXLIZET (bempedoic acid and ezetimibe) as oral ACL inhibitors for ASCVD treatment.
Emerging Paradigms in ASCVD Treatment: Diversifying Beyond Statins
Companies across the globe are diligently working toward the development of novel treatment therapies with a considerable amount of success over the years. Emerging treatments for ASCVD are currently in the process of development. These encompass IL-6 inhibitors, BET inhibitors, apoC-III inhibitors, PCSK9 inhibitors, and various other options. To date, several emerging ASCVD treatment market players include Amgen (Olpasiran), Novo Nordisk A/S (Ziltivekimab), Ionis Pharmaceuticals (Olezarsen), Resverlogix Corp (Apabetalone), and others that are developing drugs for the treatment of ASCVD.
Olpasiran, formerly known as AMG 890, is undergoing clinical trials led by Amgen and is presently in the Phase III stage for the treatment of atherosclerosis. Administered as a subcutaneous solution, Olpasiran is a small interfering RNA (siRNA) targeting apolipoprotein A. Developed utilizing dynamic polyconjugate (DPC) delivery platform technology and TRiM technology, this drug candidate is currently advancing through Phase III of its clinical development.
Ziltivekimab, a developmental drug from Novo Nordisk A/S, aims to address inflammation in cardiovascular diseases. Classified as a monoclonal antibody, it is specifically engineered to inhibit interleukin-6 (IL-6), a cytokine associated with inflammation. Research is underway to explore its potential in lowering the likelihood of cardiovascular events, especially among individuals with a history of ASCVD or other high-risk conditions. The medication is presently undergoing Phase III clinical development.
Olezarsen is an RNA oligonucleotide with antisense properties, designed to impede the synthesis of apoCII in the liver. This inhibition leads to improved clearance of triglycerides, subsequently reducing serum levels. Such an impact plays a pivotal role in diminishing the likelihood of atherosclerosis and associated cardiovascular issues. Ionis Pharmaceuticals is presently conducting Phase III clinical trials to evaluate the safety and efficacy of Olezarsen as a potential treatment for familial chylomicronemia syndrome, severe hypertriglyceridemia (sHTG), and ASCVD.
Apabetalone (RVX000222) is a prospective pharmaceutical under investigation, designed to address epigenetic processes associated with cardiovascular conditions. It functions as an inhibitor of bromodomain and extraterminal (BET) proteins, with the potential to influence gene expression linked to inflammation and lipid metabolism. Researchers are exploring its capabilities in minimizing the likelihood of significant adverse cardiovascular events among individuals at high risk. The medication is currently in Phase III of development within the Resverlogix Corp. pipeline.
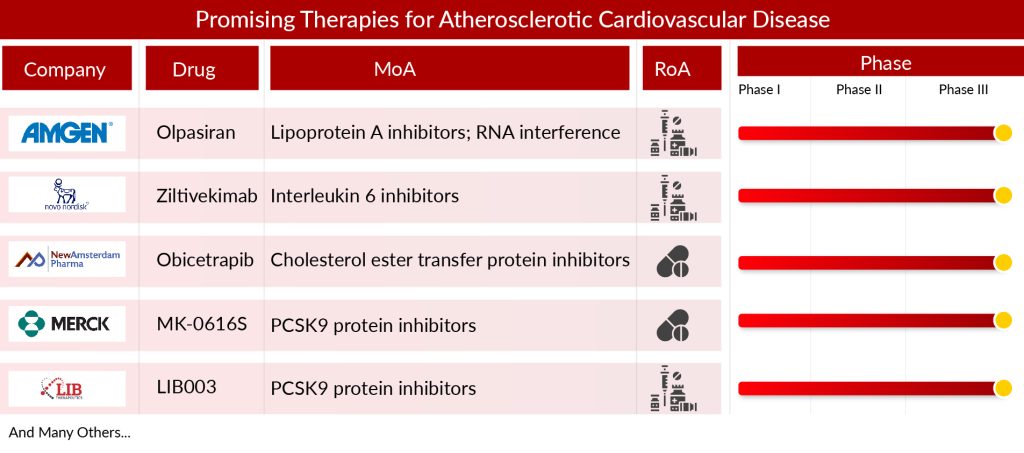
Several other assets in the ASCVD pipeline include LIB003 (Lerodalcibep) (LIB Therapeutics), CSL112 (CSL Behring), TQJ230 (pelacarsen) (Novartis Pharmaceuticals), MK-0616 (Merck Sharp & Dohme LLC), and others.
What Lies Ahead in the ASCVD Treatment Space?
In the relentless pursuit of innovative and effective strategies to combat ASCVD, the medical community is poised at the forefront of groundbreaking treatment approaches. The future of ASCVD management promises a paradigm shift, transcending traditional interventions to embrace a multifaceted and personalized approach. One of the key avenues of exploration in ASCVD treatment is precision medicine. As our understanding of the intricate interplay between genetics, lifestyle factors, and cardiovascular health deepens, tailored interventions are emerging. Genetic profiling and advanced diagnostic tools hold the potential to identify individuals at heightened risk, allowing for proactive and targeted therapeutic interventions. This personalized approach may revolutionize how we address ASCVD, moving away from the one-size-fits-all model to treatments finely tuned to an individual’s unique risk profile.
As per DelveInsight analysis, the atherosclerotic cardiovascular disease market size in the 7MM was approximately USD 23 billion in 2022 and will grow at a CAGR of 2.2% by 2032. Out of the 7MM, the United States dominated the ASCVD market in 2022, representing the largest share at 51%.
Moreover, advancements in pharmacotherapy are also shaping the landscape of ASCVD treatment. Novel medications targeting specific molecular pathways involved in atherosclerosis are under development, aiming to provide more potent and selective effects with fewer side effects. From cholesterol-lowering drugs to anti-inflammatory agents, the pharmaceutical pipeline is brimming with potential breakthroughs that could redefine the standard of care for ASCVD patients.
In the realm of interventional cardiology, minimally invasive procedures and innovative devices are playing a pivotal role in reshaping treatment approaches. Advances in catheter-based interventions, bioresorbable stents, and targeted delivery systems for therapeutic agents are at the forefront of these developments. These techniques not only offer enhanced precision in treating atherosclerotic lesions but also contribute to reduced procedural risks and improved patient outcomes.
In conclusion, the horizon of ASCVD treatment is marked by an exciting fusion of precision medicine, pharmacological innovation, technological integration, and collaborative healthcare practices. As these approaches continue to mature, the prospects for more effective, personalized, and patient-centric ASCVD management appear brighter than ever. The journey towards conquering ASCVD is a dynamic and evolving narrative, with each advancement bringing us one step closer to a future where cardiovascular health is optimized for every individual.

FAQs
According to our analysis, the total diagnosed prevalent cases of ASCVD in the 7MM were 55 million cases in 2022 and this number is predicted to climb by 2032.
Numerous non-generic medications, such as LEQVIO (inclisiran), NEXLETOL/NILEMDO (bempedoic acid), NEXLIZET/NUSTENDI (bempedoic acid and ezetimibe), PRALUENT (alirocumab), XARELTO (rivaroxaban), and REPATHA (evolocumab), are currently available in the market for ASCVD treatment.
Emerging ASCVD treatment market players include Amgen (Olpasiran), Novo Nordisk A/S (Ziltivekimab), Ionis Pharmaceuticals (Olezarsen), and Resverlogix Corp (Apabetalone), among others, which are developing drugs for the treatment of ASCVD.
According to DelveInsight analysis, the atherosclerotic cardiovascular disease market size in the 7MM was approximately USD 23 billion in 2022 and is expected to grow at a CAGR of 2.2% by 2032.
Downloads
Article in PDF
Recent Articles
- PCSK9 Inhibitors: A New Era for Cholesterol Management
- AbbVie Announces Results of Study Evaluating SKYRIZI; FDA Fast Track Designation to Arrowhead’s A...
- Arrowhead Hits the Mark in FCS Treatment with REDEMPLO Approval—Now Comes the Ionis Challenge
- Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Sclet...
- Amylyx’s AMX0114 Fast Tracked by FDA for ALS; Cellectar’s Iopofosine I 131 Granted FDA Breakthrou...