Analyzing the Key Companies and the Emerging Gene Therapy in the Dermatology Segment
Oct 22, 2021
Table of Contents
Advances in genetics research have sparked a surge in interest in gene therapy in dermatology in recent years. Dermatology treatment options have expanded beyond topical drugs to include oral medications, injectables, and light and laser therapy. Now, rather than simply treating symptoms, gene therapy in dermatology offers a new path to address the root of the disease. Expert expertise in dermatology gene therapy clinical trials, on the other hand, can be challenging to come by, as gene therapy in dermatology is still a relatively new idea. But with the continuous advancement in genome editing, gene therapy in dermatology is showing promise in several skin diseases. Many vector delivery techniques are being evaluated for efficacy and safety. At the same time, gene therapy in dermatology can have a number of drawbacks, such as immunogenicity, mutagenicity, and a lack of long-term benefits. As a result, dermatology indications are a promising area of focus for future gene therapy in dermatology.
Leading Companies in Gene Therapy in Dermatology Domain
Gene therapy in dermatology is frequently on an accelerated development pathway due to the small patient populations available for dermatology gene therapy trials and the high unmet need. Major pharmaceutical companies in gene therapy in dermatology include Krystal Biotech, Castle Creek Biosciences, Almirall, Tyris Therapeutics, Abeona Therapeutics, Fibrocell Science, Amryt Pharma, Sterna Biologicals, Temprian Therapeutics, Holostem Terapie, Azitra, Novartis, Avita Medical, and others. These companies are exploring gene therapy in dermatology sector with all their experience and expertise and are trying their best to develop novel drugs. The details of some of the pharmaceutical companies, along with their lead products, are listed below:
Downloads
Click Here To Get the Article in PDF
Krystal Biotech
Krystal Biotech, Inc. is a clinical-stage gene therapy company using its proprietary Skin TARgeted Delivery (STAR-D) platform to develop effective and innovative treatments for skin diseases. They are initially developing topical and intradermal “off-the-shelf” novel therapies for rare and orphan dermatological indications and expanding the use of pioneering STAR-D technology to target and treat other skin conditions.
Lead Therapy:
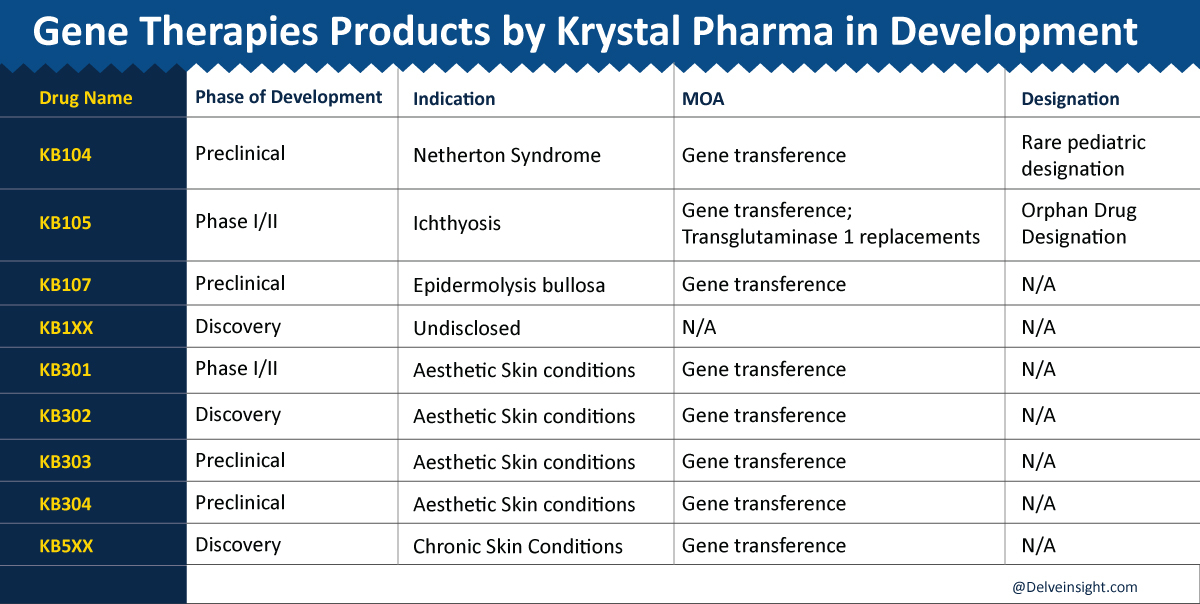
Beremagene geperpavec (“B-VEC,” previously “KB103”) is Krystal’s lead product candidate that seeks to use gene therapy for all forms of dystrophic epidermolysis bullosa treatment.
Technology & Manufacturing:
B-VEC uses Krystal’s STAR-D technology to deliver functional human COL7A1 genes directly to the skin of affected patients. The platform consists of an engineered HSV-1 vector and skin optimized gene transfer technology, suitable for introducing into the patient one or more therapeutic genes relevant to treating skin disease. The modified HSV-1 is a replication-defective, non-integrating viral vector that can efficiently penetrate a broad range of skin cells.
The COL7A1 genes then express functional collagen VII to form anchoring fibrils, thus stabilizing the patient’s otherwise fragile skin. The therapy is applied topically to DEB wounds. B-VEC is manufactured in-house using the commercial process and at scale in Krystal’s fully functional GMP ANCORIS facility, located near corporate headquarters in Pittsburgh.
Clinical Stage:
The drug is currently in the Phase III stage of development. The top-line data and BLA filing are anticipated in 2021; EMA aligned on pivotal study design, and an MAA is expected shortly after BLA.
Regulatory:
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have each granted B-VEC orphan drug designation for DEB treatment, and the FDA has granted B-VEC fast track designation and rare pediatric designation for the treatment of DEB. In addition, in 2019, the FDA granted Regenerative Medicine Advanced Therapy (RMAT) to B-VEC for the treatment of DEB, and the EMA granted PRIority MEdicines (PRIME) eligibility for B-VEC to treat DEB.
Castle Creek Biosciences
Castle Creek Biosciences, Inc., a clinical-stage cell and gene therapy company, focused on developing and commercializing disease-modifying therapies for patients suffering from rare diseases for which there is a lack of available treatment options. The company’s approach to personalized biologics is distinctive based on our proprietary, autologous fibroblast technology platform. By extracting fibroblast cells from a patient’s skin, they create localized treatments that are compatible with the unique biology of each patient and have the potential to address the underlying cause of disease.
Therapy 1:
D-Fi (dabocemagene autoficel, formerly known as FCX-007) is being developed as a disease-modifying, autologous cell-based gene therapy to address the deficiency of functional type VII collagen protein (COL7) in patients with autosomally recessive or dominant dystrophic epidermolysis bullosa or DEB.
Technology:
D-Fi comprises autologously derived dermal fibroblasts genetically modified with a lentiviral vector containing the COL7A1 gene to express COL7. D-Fi is locally administered by injection directly into the papillary dermis of wounds of DEB, where the COL7 protein can support the formation of anchoring fibrils in the skin, thereby avoiding systemic treatment.
Clinical Stage:
The company’s dystrophic epidermolysis bullosa drug candidate, D-Fi is currently in the most advanced stage i.e., Phase III stage of development.
Regulatory:
The U. S. Food and Drug Administration has granted Orphan Drug designation to D-Fi to treat Dystrophic Epidermolysis Bullosa, including RDEB. In addition, D-Fi has been granted Rare Pediatric Disease designation, Fast Track designation, and Regenerative Medicine Advanced Therapy (RMAT) designation by the FDA for recessive dystrophic epidermolysis bullosa treatment.
Therapy 2:
FCX-013 is being developed as a disease-modifying, autologous cell-based gene therapy that utilizes lentiviral vectors to deliver functional matrix metalloproteinase 1 genes (MMP-1) to patients with moderate to severe localized scleroderma. MMP-1 encodes the enzyme responsible for breaking down collagen.
Technology:
FCX-013 incorporates a biologic switch activated by an orally administered compound (veledimex) to control protein expression at the site of the localized scleroderma lesions. FCX-013 is designed to be injected under the skin at the location of the fibrotic lesions where the genetically modified fibroblast cells will produce MMP-1 to break down excess collagen accumulation. With the FCX-013 therapy, the patient will take an oral compound (veledimex) to facilitate protein expression. Once the fibrosis is resolved, the patient will stop taking the oral compound, which will control further MMP-1 production.
Clinical Stage:
The drug FCX-031 is currently in Phase I/II clinical trial stage.
Regulatory:
The US Food and Drug Administration has granted Orphan Drug designation to FCX-013 for the treatment of localized scleroderma. In addition, FCX-013 has been granted Rare Pediatric Disease designation and Fast Track designation for moderate to severe localized scleroderma treatment.
Collaboration and Deals:
In September 2021, Castle Creek Biosciences announced a research collaboration with Mayo Clinic to advance the discovery and pre-clinical development of investigational gene therapy candidates for the treatment of osteogenesis imperfecta (OI) and classical Ehlers-Danlos syndrome (EDS), which are rare genetic connective tissue disorders that currently have no treatments approved by the U.S. Food & Drug Administration (FDA). The research is being led by principal investigator David R. Deyle, M.D., a board-certified medical geneticist with the department of medical genetics at Mayo Clinic and a leader in the field of connective tissue disorders.
Abeona Therapeutics
Abeona Therapeutics is a fully integrated gene and cell therapy company at the forefront of the rapidly advancing field of genetic medicine. The company’s expertise across R&D, manufacture, and discovery of the novel gene and cell therapies has uniquely positioned to bring new drugs to patients in need.
Lead Therapy:
Abeone’s lead asset EB-101 is an autologous, gene-corrected cell therapy for RDEB, a rare connective tissue disorder without an approved treatment in which patients suffer from severe epidermal wounds that impact the length and quality of their lives. People with RDEB have a defect in the COL7A1 gene, leaving them unable to produce Type VII collagen that helps anchor the dermal and epidermal layers of the skin.
Technology:
Treatment with EB-101 involves using gene transfer to deliver COL7A1 genes into an RDEB patient’s skin cells (keratinocytes) and transplanting them back to the patient to enable normal Type VII collagen expression and skin function.
Clinical Stage:
Investigators are currently enrolling the VIITAL™ Study, a pivotal Phase III clinical trial evaluating EB-101 for the treatment of RDEB patients. EB-101 has the potential to be the first approved therapy for RDEB and the only durable treatment to address large chronic wounds, which are the most painful and debilitating. In a Phase I/II clinical trial, EB-101 was well-tolerated, resulting in significant and durable wound healing, with up to 5 years of follow-up. Continuous Type VII collagen expression was observed more than two years after treatment, and no drug-related serious adverse events have been observed to date.
Regulatory:
Abeona holds several regulatory designations in the U.S. and EU for EB-101 that allow increased interactions and guidance from the FDA and EMA. In the U.S., these include Regenerative Medicine Advanced Therapy, Breakthrough Therapy, and Rare Pediatric Disease designations, and Orphan Drug designation in both the U.S. and EU.
Amryt Pharma
Amryt is a global commercial-stage biopharmaceutical company focused on acquiring, developing, and commercializing innovative treatments to help improve the lives of patients with rare and orphan diseases. Amryt comprises a strong and growing portfolio of commercial and development assets. Amryt’s pre-clinical gene therapy candidate, AP103.
Lead Therapy:
AP103 offers a potential treatment for patients with Dystrophic EB, and the polymer-based delivery platform can be developed to treat other genetic disorders. AP103 is a topical gene therapy intervention to restore the expression of the COL7A1 gene. – In vitro tests of RDEB keratinocytes, the main cell type in the top layer of skin, showed that a single delivery of the human collagen VII gene, by AP103, restored collagen VII production to levels exceeding those produced by healthy human keratinocytes – Topical application of AP103 onto a 3-D matrix of human RDEB skin restored collagen VII along the basement membrane to levels similar to those observed post-delivery using a single vector – AP103 exhibited no evidence of cellular toxicity after repeated administration.
Technology:
The Company’s in-licensed gene therapy platform has completed two pre-clinical studies which showed that topical application of AP103 restored production of collagen VII in pre-clinical models of EB to levels exceeding those produced by healthy human keratinocytes (cells that regenerate the outer layer of the skin) and to levels similar to those observed following delivery of the same gene with a viral vector. Restricting the production of collagen VII in skin cells could be transformative for these patients, potentially making their skinless fragile, and more resistant to damage and blistering.
Clinical Stage:
In January 2019 announced results from these studies which support the development of AP103 as a potentially disease-modifying therapy for patients with RDEB. RDEB is a particularly severe form of Epidermolysis Bullosa and is caused by mutations in a single gene, COL7A1, which codes for the production of collagen VII, a structural protein vital for the elastic and structural integrity of the skin.
Regulatory:
Amryt pharma’s lead asset AP103 received the Orphan Drug Designation by the US FDA in December 2020 for the treatment of dystrophic epidermolysis bullosa.
Holostem Terapie
Holostem Terapie Avanzate is the first biotechnological company entirely devoted to developing, manufacturing, registering, and distributing Advanced Therapies Medicinal Products (ATMPs) based on cultures of epithelial stem cells cell and gene therapy. The main aim of Holostem Terapie Avanzate is to promote epithelial stem cell-based Regenerative Medicine for patients with no alternative therapeutic solutions. Holostem Terapie Avanzate is a university spin-off founded in 2008 through the profitable union among the scientific know-how of internationally renowned researchers such as Michele De Luca and Graziella Pellegrini, the innovative spirit of the University of Modena and Reggio Emilia, and the industrial know-how of Chiesi Farmaceutici S.p.A., a leading pharmaceutical company in Italy.
Product Portfolio:
Holostem Terapie Avanzate is currently running two Phase I/II clinical trials for combined ex-vivo cell and gene therapy for Recessive Dystrophic EB and Col17-JEB, implementing a Phase I/II clinical trial for laminin 332-JEB and developing new gene-editing technologies for the treatment of dominant forms of EB, in collaboration with the Centre for Regenerative Medicine “Stefano Ferrari.”
Avita Medical
AVITA Medical is a regenerative medicine company that has developed a technology platform to address unmet medical needs in therapeutic skin restoration. Avita is exploring the use of the RECELL System technology platform for gene therapy involving genetically modified skin cells related to skin disorders such as genodermatoses and rejuvenation. Currently, AVITA Medical and scientists at the Gates Center for Regenerative Medicine at the University of Colorado, School of Medicine are collaborating on preclinical research to establish proof of concept and explore further development of a spray-on treatment of genetically modified cells for patients with EB, with potential applicability to other genetic skin disorders. The partnership pairs AVITA Medical’s patented and proprietary Spray-On Skin™ Cells technology and expertise with the Gates Center’s innovative, patent-pending combined reprogramming and gene-editing technology to allow cells to function correctly.
Azitra
Azitra is a clinical-stage medical dermatology company that creates and develops novel products to treat skin conditions and diseases by leveraging its extensive knowledge of proteomics, genetic engineering, and the skin microbiome.
Technology:
The Company’s technology platform is based on a proprietary strain of the natural commensal bacteria Staphylococcus epidermidis, developed in collaboration with scientists at Yale University and the Jackson Laboratory. Simply applying these commensal bacteria to the skin improves its appearance, hydration, and healing.
Lead Therapy:
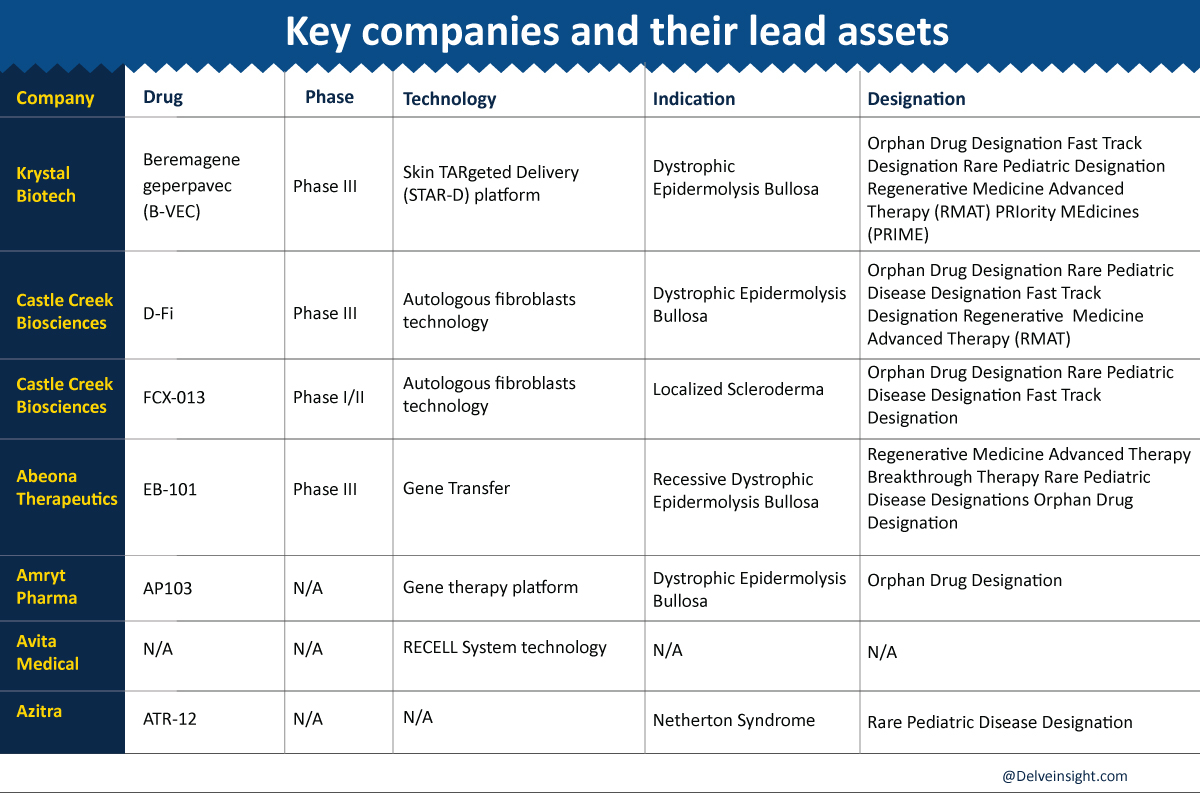
ATR-12 is a proprietary strain of Staphylococcus epidermidis engineered to express therapeutic levels of LEKTI protein to treat Netherton syndrome (NS). ATR-12 will address an unmet need in an annual market that exceeds $1.5B. Azitra believes that an ideal Netherton Syndrome treatment would address the key drivers of Netherton Syndrome disease—i.e., simultaneous skin barrier deficiency, microbial dysbiosis (microbiome), and an activated cutaneous immune response—as opposed to symptomatic relief. ATR-12 is provided as a non-aqueous ointment for topical application and is designed to inhibit the overactive proteases responsible for NS.
Regulatory:
The US FDA has granted ATR-12 Rare Pediatric Disease Designation for pediatric diseases affecting a limited number of patients in May 2020.
Way Ahead
The future of gene therapy in dermatology is very prominent as several pharmaceutical and biotech companies are involved in developing novel gene therapies products for various skin diseases. Several novel drugs are already in the dermatology gene therapy pipeline and the research is still on. It will open new pavements for the smaller companies as well. Currently, the gene therapy in dermatology domain is consolidated with several technologies such as STAR-D technology, Autologous fibroblast technology, RECELL System technology, and others but the biotech companies are still engaging themselves in the quest for developing new technologies that can be the game-changer in the domain. With the promising outlook of the dermatology gene therapy pipeline landscape along with the active participation of leading companies, we may witness many more advancements in gene therapy in dermatology in the near future.
The leading pharmaceutical and biotech companies such as Krystal Biotech, Castle Creek Biosciences, Almirall, Tyris Therapeutics, Abeona Therapeutics, Fibrocell Science, Amryt Pharma, Sterna Biologicals, Temprian Therapeutics, Holostem Terapie, Azitra, Novartis, Avita Medical, and others are diligently working towards developing gene therapy products for several skin diseases.
Several companies are working on their lead assets for various skin diseases. The novel drugs that are in pipeline for dermatological diseases include B-VEC, D-Fi, FCX-013, EB-101, AP103, ATR-12, along with others.
Gene therapy is a method that alters the biological characteristics of live cells or affects the expression of a person’s genes for therapeutic purposes. Cancer, hereditary disorders, and infectious diseases are among the diseases for which gene therapy products are being investigated.
Gene therapy is intended to transfer genetic material into cells in order to compensate for defective genes or to produce a useful protein. In most cases, a gene that is introduced directly into a cell does not function. To transmit the gene, a carrier called a vector is genetically created.
Skin is an ideal organ for gene therapy because it is a superficial, easily manipulatable, visible, and flexible organ. The dermis is a highly vascular structure that can manufacture and secrete a large number of proteins into the circulation.
Downloads
Article in PDF