Sarepta’s ELEVIDYS: First Gene Therapy for Duchenne Muscular Dystrophy (DMD) Treatment
Jun 30, 2023
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disease that affects 1 in every 3,500 to 5,000 male neonates worldwide. It is caused by mutations in the gene encoding the protein dystrophin. These genetic changes emerge as developmental delays and, in more severe types of DMD, limb weakness, loss of independence, and respiratory issues. Furthermore, it is estimated that 250K people in the United States suffer from muscular dystrophy.
As per DelveInsight analysis, the total number of prevalent cases of DMD in the seven major markets was around 31K in 2023. In the United States, in 2023, the highest proportion of age-specific cases was observed in children aged 5- 9, followed by those aged 10-14 and 15-20.
Although there is no cure for any form of muscular dystrophy, treatment for some forms of the condition, including DMD, can assist extend a person’s mobility and improve heart and lung muscle strength. Gene replacement or other genetic therapies linked to specific mutations to restore dystrophin production, membrane stabilization and/or upregulation of compensatory proteins, and reduction of the inflammatory cascade and/or enhancement of muscle regeneration are the main therapeutic strategies for DMD treatment.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Cell and Gene Therapies in Rare Disorders: From Rarity to Recovery
- Anbogen’s ABT-301 Cleared by FDA for Phase I/II Colorectal Cancer Trial; Dyne’s DYNE-251 Gets FDA...
- What Does the Future Hold for Gene Therapy in the Duchenne Muscular Dystrophy (DMD) Treatment Mar...
- Is the Cure for Duchenne Muscular Dystrophy in the Pipeline?
- Duchenne Muscular Dystrophy Market: What’s More Beyond Exon-Skipping Therapies?
The path to gene therapy for genetic disorders has been long and costly, but the DMD treatment space received some good news. On June 22, 2023, the FDA approved Sarepta’s ELEVIDYS, the first gene therapy for Duchenne muscular dystrophy treatment. Sarepta and Roche entered into a collaboration in December 2019, with Roche paying $1.15 billion upfront for exclusive rights to SRP-9001. It is FDA’s 13th gene therapy approval since 2017, and the first to address a prevalent genetic disease in ambulatory pediatric patients aged 4 through 5 years. ELEVIDYS, a one-time treatment, will cost $3.2 million.
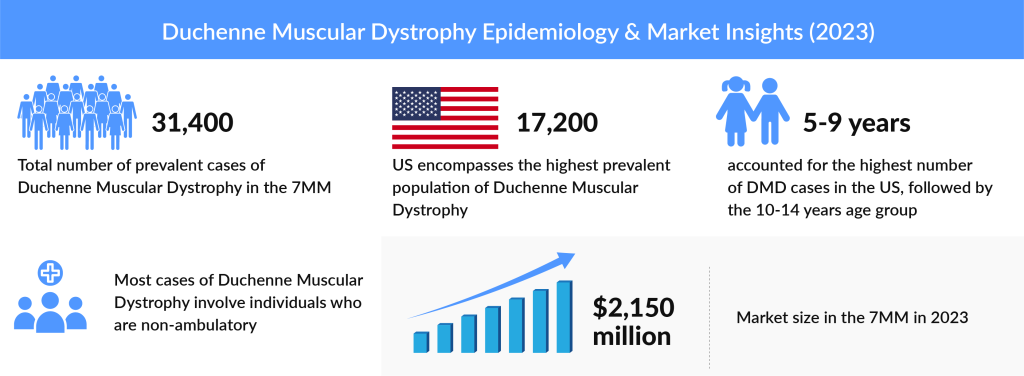
The 5-9 years age group accounted for the highest cases, followed by 10-14 years in 2023 in the US.
The decision was postponed in late May after FDA officials and consultants expressed reservations about the strength of Sarepta’s findings thus far; SRP-9001 appears to have just a minor effect on muscular performance, and only in some persons.
“The approval of ELEVIDYS marks a turning point in the treatment of Duchenne. ELEVIDYS is the first and only gene therapy approved for Duchenne, and this approval brings us closer to our goal of bringing forward treatment with the potential to alter the trajectory of this degenerative disease,” said Doug Ingram, Sarepta’s president, and CEO. “As we prepare to launch ELEVIDYS, we should recognize and celebrate the decades of dedication and hard work put in by the patient community, families, clinicians, and our Sarepta colleagues that resulted in today’s approval. Our confirmatory trial, EMBARK, is expected to be completed in the fourth quarter of this year. If EMBARK confirms the advantages demonstrated in our previous trials, Sarepta will submit a BLA supplement as soon as possible to broaden the authorised label as much as good science allows.”
ELEVIDYS addresses the core genetic cause of Duchenne, mutations in the dystrophin gene that result in a lack of dystrophin protein, by delivering to muscle cells an ELEVIDYS micro-dystrophin gene that codes for a shorter form of dystrophin. This fast approval is predicated on an increase in skeletal muscle ELEVIDYS micro-dystrophin protein expression. In addition to efficacy data from two clinical studies, SRP-9001-102 and SRP-9001-103, and safety data from SRP-9001-101, SRP-9001-102, and SRP-9001-103, ELEVIDYS is backed by biologic and empirical evidence. The company has agreed to complete a confirmatory trial in accordance with the accelerated approval procedure. EMBARK, the global, randomized, double-blind, placebo-controlled Phase III study for ELEVIDYS, is now completely enrolled, with top-line data expected in late 2023.
In April 2024, Sarepta forecast flat sales for its new gene therapy, ELEVIDYS, leading to a first-quarter sales estimate of $131 million. As noted by Mizuho Securities analyst Uy Ear, despite analyst expectations of $138 million, Sarepta’s stock fell by almost 11% following this guidance.

The Other DMD Gene Therapies in the Race
With ELEVIDYS BLA approval, Roche and Sarepta have taken the lead in a close race to bring a gene therapy for DMD treatment across the regulatory finish line. Pfizer is waiting in the wings after its DMD candidate PF-06939926 hit a snag in late 2021 when a treated patient died. The FDA quickly placed the trial on clinical hold, which was later lifted when the company addressed the agency’s concerns. In 2020, PF-06939926 was given Fast Track status. Pfizer is currently undertaking a phase III trial in 99 boys with their version of microdystrophin in a comparable AAV vector, with preliminary results expected next year.
Several other companies are working on their own microdystrophin, including Regenxbio in Rockville, Maryland, which launched a small study early this year. Recently, in April 2023, Regenxbio announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for RGX-202, a potential one-time gene therapy for Duchenne muscular dystrophy treatment. In a heartbreaking turn of events in May 2024, a young boy tragically lost his life to cardiac arrest during Pfizer’s Phase II gene therapy trial for Duchenne muscular dystrophy. This devastating incident prompted Pfizer to halt dosing in its ongoing Phase III CIFFREO trial, casting a shadow over the future of this promising treatment.
Other companies are working to improve the viral vector that carries the gene. Solid Biosciences terminated the phase II trial of its own microdystrophin formulation in 2022 to transition to a different vector that particularly targets muscle cells and may likely be administered at lower doses. Later in 2023, the business intends to begin dosing patients. Chamberlain also presented a new method combining several AAV vectors that each contain a piece of the dystrophin gene at the American Society for Gene & Cell Therapy’s annual meeting in Los Angeles in May 2023. In June 2024, Genethon, a non-profit gene therapy organization, provided updates on its DMD clinical trials and advances in gene therapies for limb-girdle muscular dystrophies.
Other researchers are investigating other vectors such as lipid capsules, nanoparticles, antibodies that deliver dystrophin genes to muscle cells, and viruses that integrate into a patient’s genome. In theory, these medicines might be given to those who have already had AAV gene therapy.
However, now that an approved therapy is available, companies testing their own therapies may find it more difficult to recruit trial participants, who may not want to chance receiving a placebo. Furthermore, Reshma Ramachandran, a health-services researcher at Yale University in New Haven, Connecticut, is concerned that if companies know they can get a microdystrophin therapy approved, they will be more likely to develop their own slightly tweaked versions rather than spending more time developing innovative approaches of their own.
Future Outlook of DMD Gene Therapy Space
Gene therapy development has always been a risky and expensive business, and the recent economic slump has resulted in the closure of numerous biotech companies. Nonetheless, the FDA has approved more gene therapies each year since its first, Kymriah for leukemia, in 2017. However, approval of a DMD gene treatment would still be a significant step forward. Until date, the majority of gene therapies that have been approved have targeted tumors, highly rare diseases, and problems such as retinal disorders, which are straightforward to target with a virus.
The future outlook for DMD gene therapy treatment space is promising. Researchers and scientists are actively working on refining existing gene delivery systems and developing new ones that can efficiently deliver the therapeutic genes to muscle tissues throughout the body. The goal is to develop gene therapies that provide long-term and sustainable benefits, allowing individuals with DMD to lead more functional and independent lives.
Additionally, advancements in gene editing technologies, such as CRISPR-Cas9, offer another avenue for DMD treatment. CRISPR-based gene editing holds the potential to correct the genetic mutation responsible for DMD directly within a patient’s cells. While still in the early stages of development, preclinical studies using CRISPR-Cas9 for DMD show promise and could potentially provide a more precise and targeted approach to treating the condition.
However, it’s important to note that despite the significant progress made, there are still challenges to overcome. Ensuring the safe and efficient delivery of therapeutic genes to all affected muscles, addressing potential immune responses to the viral vectors or gene editing tools, and achieving widespread accessibility and affordability of gene therapies remain key areas of focus for researchers and healthcare professionals.

Downloads
Article in PDF
Recent Articles
- Sarepta Secures FDA Approval of Revised Prescribing Information for ELEVIDYS; Merck to Acquire Ci...
- Gene therapy: Following Pfizer’s Unexpected Failure, Sarepta is celebrating its much-awaite...
- Bristol-Myers Squibb’s Opdivo & Yervoy Combo Trial; Sarepta’s Gene Therapy SRP-9001 for DMD;...
- Sarepta Therapeutics Pauses ELEVIDYS Shipments in U.S. After FDA Intervention Over Patient Deaths...
- Pfizer tightens DMD trial criteria over safety concerns; Kytopen raises $30M; Eli Lilly taps Reif...



