Examining the Therapeutic Advances in Fabry Disease Treatment Landscape
Mar 28, 2022
Table of Contents
Fabry Disease is an inherited lysosomal storage disease caused by a nonfunctional or partially functional enzyme called alpha galactosidase A (α-Gal A). Decreased activity of α-Gal A in lysosomes results in the accumulation of enzyme substrates (Gb3 and lyso-Gb3) which cause cellular damage in tissues throughout the body. Currently, there is no permanent cure for Fabry Disease. But, recent developments in the Fabry Disease treatment scenario are leading to a symptomatic cure, relieving the condition. Fabry Disease symptoms include pain that spreads through the body (called a Fabry crisis), gastrointestinal complications, headaches, impaired sweating, vertigo, and hearing impairment.
It was once thought that Fabry Disease inheritance only affected males and females were considered to be the “carriers.” However, it is now known that both men and women can get Fabry Disease, though it may manifest differently depending on gender. Despite being X-linked, heterozygous women may experience all the Fabry Disease signs and symptoms that are also seen in men. But when compared with hemizygous males, Fabry Disease symptoms in females typically emerge at an older age and with less severity.
Downloads
Click Here To Get the Article in PDF
For Fabry Disease diagnosis, a GLA gene test can be performed. This exclusive Fabry Disease test aims at measuring the level of the alpha-GAL enzyme. If the alpha-GAL enzyme assay shows low enzyme activity, then the person is considered to have experience Fabry disease onset. Males with classic Fabry disease essentially have no alpha-galactosidase A enzyme (less than 1% of normal). Whereas, for females, a DNA test for Fabry Disease diagnosis is the most prescribed. Fabry Disease can be classified into two forms – Classic Fabry Disease and Late onset Fabry Disease.
Fabry Disease Epidemiology and Statistics
Fabry Disease is an extremely rare and inherited genetic disorder that can lead to life-threatening heart attacks and kidney problems such as kidney failure. This disorder belongs to a group of diseases known as lysosomal storage disorders. It affects one in many, and Fabry Disease incidence is observed in both the genders. As per DelveInsight, Fabry Disease prevalence in the 7MM countries was estimated to be 13,050 cases in the year 2020. Out of which, the United States was observed to have the highest Fabry Disease prevalence, where males accounted for 4,111 cases and 3,820 females were affected with Fabry Disease. DelveInsight estimates that the number will increase by 2030.
In 2020, among the 7MM countries, there were 7,931 prevalent cases of Fabry Disease in the United States. The EU5 countries accounted for 4,682 cases, whereas Japan had a total of 437 Fabry Disease cases. Among EU5 countries, the UK had the highest Fabry Disease population which was 1,113 cases, the same year. Fabry Disease epidemiology segmentation can be done on the basis of prevalent cases of Fabry Disease, Gender-specific cases of Fabry Disease, Phenotype-specific cases of Fabry, and Disease and Age-specific cases of Fabry Disease.
Fabry Disease Market Insights
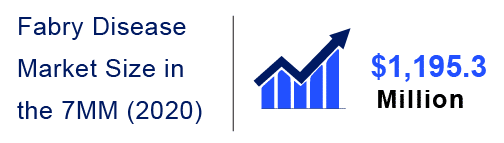
In recent years, factors such as increasing Fabry Disease prevalence, currently marketed therapies, along the promising emerging pipeline therapies with new mechanisms of action, the launch of these potential therapies are all anticipated to fuel the Fabry Disease market in the coming years. According to DelveInsight, the Fabry Disease market size in the 7MM countries was estimated to be USD 1,195.3 million in the year 2020.
Many key pharmaceuticals that are proactively involved in producing Fabry Disease drugs in the coming years include names such as Protalix Biotherapeutics, Sanofi Genzyme, Idorsia Pharmaceuticals, Avrobio, Sangamo Therapeutics, Shire, Takeda, Amicus Therapeutics, Ozmosis Research Inc, CellGenTech, Inc, uniQure, Codexis, Canbridge, Eleva GmbH, MP6 therapeutics, Sigilon Therapeutics, 4D Molecular Therapeutics, and Freeline Therapeutics, whose key Fabry Disease treatment therapies are expected to be launched in the coming years and can also serve as a major booster for Fabry Disease treatment market.

Current Fabry Disease Treatment Landscape
The market of Fabry Disease treatment is continuously evolving due to the presence of many existential therapies as well as several new therapies that are under research. Currently, there are only a few FDA-approved Fabry Disease treatment therapies, the first one is Sanofi Genzyme's enzyme replacement therapy (ERT) with Fabrazyme (agalsidase beta). It has been the standard of care for Fabry Disease treatment since the year 2001. Fabrazyme can be considered as the only enzyme replacement Fabry Disease medication that has been FDA approved and has long-term efficacy and safety data. It received accelerated approval from the FDA in 2003. 18 years later, the FDA converted the enzyme replacement therapy treatment authorization to full, regular approval.
Replagal (agalsidase alfa), developed by the Shire, received European Commission approval as an Orphan Drug in August 2001 and in February 2007 in Japan with the marketing rights with Dainippon Sumitomo Pharma. It is currently approved as a Fabry Disease treatment therapy in almost38 countries but not in the US. Also, in addition to that is a Fabrazyme biosimilar agalsidase beta BS developed by JCR Pharmaceuticals and GlaxoSmithKline has been available in Japan since 2018. It has a pricing advantage over Fabrazyme and Replagal, the only Japan-originated therapeutic.
Though enzyme replacement therapy (ERT) has several benefits, it also has serious limitations. These include an inconvenient dosing schedule every 2 weeks causing variation in enzyme levels, risk of infusion-related reactions and infections, and the possibility of developing antibodies against Fabry Disease treatment. To counteract these limitations, Amicus Therapeutics developed Galafold which has been the most recent entrant in the Fabry Disease market for the last 15 years.
This Fabry Disease treatment therapy gained significant market share from the existing treatments as it alleviated the current unmet needs. Galafold is what researchers and scientists call a chaperone therapy, and is also the first of this class of medications. It represents better value for money and may also provide more clinical benefits to Fabry Disease patients. Galafold, however, is only eligible to patients with amenable mutations i.e. 35–50% of the overall Fabry Disease population. It is concluded that, for Fabry Disease, one-size-fits-all treatment is non-existent as of now.
Although a number of novel and potential new therapies are under research, including new forms of ERT, substrate reduction therapy, mRNA therapy, and gene therapy to address the current challenges in the Fabry Disease treatment landscape.
Emerging Drugs in Fabry Disease Treatment Pipeline
In order to combat the current unmet needs of the Fabry Disease treatment market and to provide better treatment options, several major pharma players are working aggressively to develop new Fabry Disease therapies with a novel mechanism of action. Emerging therapies such as Pegunigalsidase alfa (Protalix Biotherapeutics), Venglustat (Sanofi Genzyme), Lucerastat (Idorsia Pharmaceuticals), AVR-RD-01 (Avrobio), ST-920 (Sangamo Therapeutics), 4D-310 (4D Molecular Therapeutics), and FLT190 (Freeline Therapeutics), and others are expected to enter the Fabry Disease treatment market in the coming years.
There are many Fabry Disease pipeline drugs in different phases of development under clinical trials, which include -
PRX-102 or Pegunigalsidase Alfa is an experimental Enzyme Replacement Therapy (ERT) that is currently under development by Protalix Biotherapeutics for Fabry Disease treatment. It is being developed using Protalix’s plant-based ProCellEx platform, a plant cell culture expressed, and a chemically modified version of the recombinant α-GAL A protein. The company has completed three Phase III studies (BALANCE, BRIGHT, and BRIDGE). The final outcomes of the BALANCE study will be out in Q1 of 2022. In May 2020, the companies filed an application with the US Food and Drug Administration seeking the accelerated approval of PRX-102, given at a dose of 1 mg/kg every other week, to treat adults with Fabry. After granting it priority review, the agency rejected the application in April 2021 due to issues with facility inspections and manufacturing processes, partially caused by travel restrictions during the COVID-19 pandemic. The decision was not associated with concerns related to the therapy’s safety or effectiveness shown in clinical trials.
AVR-RD-01 is an investigational lentiviral-based gene therapy developed by Avrobio for Fabry Disease treatment. It is a gene delivery system using a harmless virus that inserts the nonmutated form of the GLA gene containing information for making functional α- GAL A into the body. AVR-RD-01 is currently being tested in Phase I/II. To support the use of AVR-RD-01 in a broad Fabry Disease population, the company expects to include female patients, eliminate antibody status exclusions and collect additional cardiovascular and CNS data in the FAB-GT trial. Avrobio is anticipated to plan a pivotal trial in order to support an FDA approval of AVR-RD-01 as first-line gene therapy for Fabry Disease treatment. If the FDA is under the agreement, this drug will be a fine competitor for Sanofi Genzyme’s Fabrazyme.
Venglustat (also known as ibiglustat) is an orally administered small molecule, currently being investigated by Sanofi Genzyme as another potential Fabry Disease drug. Also, this drug is developed for the treatment of several other rare diseases as well. It is an inhibitor of an enzyme called glucosylceramide synthase (GCS) and modifies the enzyme substrates. GCS turns its substrate, ceramide, into glucosylceramide (GL-1) during lipid metabolism, a series of biochemical reactions that degrade and generate lipids. The molecule is in the Phase III stage of development for Fabry Disease treatment therapy. The company expects to file submission by the year 2025.
ST-920 is a gene therapy under investigation by Sangamo Therapeutics. It is a gene therapy that comprises an AAV vector carrying a GLA gene construct driven by a proprietary liver-specific promoter. It is designed to enable a patient’s liver to produce a long-lasting and continuous supply of the α-Gal A enzyme. Preclinical studies of ST-920 showed a positive response, and based on the positive response, the FDA accepted the IND application of the gene therapy for Fabry Disease treatment, and the company initiated the Phase I/II clinical study of the product.
FLT190 is a next-generation gene therapy that is under development by Freeline Therapeutics. It is a next-generation gene therapy that uses an adeno-associated virus (AAV8) as a vehicle to deliver a healthy copy of the GLA gene in the hopes that it will induce the production of normal α-GAL A. Preclinical data using a gene therapy platform for expression of the enzyme α-galactosidase A (GLA) has demonstrated safety and efficacy in various animal models. Based on these company initiated a Phase I/II trial to evaluate FTL190 for Fabry Disease treatment. The interim data readouts are expected in 2022. It has also received orphan designation by the EMA.
4D-310 is a gene medicine engineered with a proprietary optimized Adeno-associated virus (AAV) capsid discovered by 4D Molecular Therapeutics through its proprietary Therapeutic Vector Evolution platform. It holds promise for Fabry Disease treatment by using an AAV vector to deliver a functional copy of the GLA gene, resulting in α-GAL A production in target tissues. This Fabry Disease drug received Fast Track Designation from the FDA in the third quarter of 2020.
Idorsia Pharmaceuticals is developing a Lucerastat, a glucosylceramide synthase inhibitor, as a substrate reduction therapy for Fabry Disease treatment. It is an orally bioavailable small-molecule and low molecular weight iminosugar that inhibits glucosylceramide synthase and can provide substrate reduction. It offers a new treatment approach for people with Fabry Disease irrespective of their genetic mutation type.
Moss-aGal is an investigational Fabry Disease treatment therapy being developed by Greenovation Biotech GMBH, it is a recombinant form of human alpha-galactosidase produced in moss (physcomitrella patens) using Greenovation’s BryoTechnology. Expression in moss creates a protein that is identical to the human protein with customized glycosylation patterns. The production is free of human pathogens and animal compounds. The production is free of human pathogens and animal compounds. The company has completed the Phase I trial of the product with positive results.

Unmet Needs in Fabry Disease Treatment Domain
There are several unmet needs still present in the Fabry Disease treatment landscape. It might include several factors such as many challenges faced during the diagnosis of Fabry Disease, the limitations which are found in the ongoing gene therapies used for treatment. Due to being a rare disease, the understanding of Fabry Disease is very poor, also, there is a need to develop more efficient clinical biomarkers for disease testing, with a novel Fabry Disease mechanism of action.
Way Ahead
Current Fabry Disease treatment market landscape is devoid of many therapies apart from those working on the basis of Enzyme Replacement Therapy. ERT definitely has changed the outcome of treatment in the Fabry Disease population, however, the enzyme must be delivered intravenously, which can be challenging as it requires repeated calculations. Although ERT is generally tolerated well, there is room for improvement, and to overcome these limitations, chaperone therapy is recently been introduced for Fabry Disease treatment as a new-age molecular therapeutic approach to protein misfolding diseases.
Frequently Asked Questions (FAQs)
Fabry Disease is an inherited lysosomal storage disease caused by a nonfunctional or partially functional enzyme called alpha galactosidase A (α-Gal A). Decreased activity of α-Gal A in lysosomes results in the accumulation of enzyme substrates (Gb3 and lyso-Gb3) which cause cellular damage in tissues throughout the body.
Currently, there is no permanent cure for Fabry Disease. But, recent developments in the Fabry Disease treatment scenario are leading to a symptomatic cure, relieving the condition.
Fabry Disease is rare, inherited, and a serious genetic disorder. It is a lipid storage disorder that can cause long-term difficulties in the kidneys, heart, and nervous system. Fabry Disease can be understood as a terminal illness as well.
Fabry Disease involves many potential life-threatening complications such as progressive kidney damage, heart attack, and stroke. It can be fatal.
Downloads
Article in PDF