Roche’s GAZYVA Extends Indications Into Lupus, Strengthening Immunology Pipeline
Oct 27, 2025
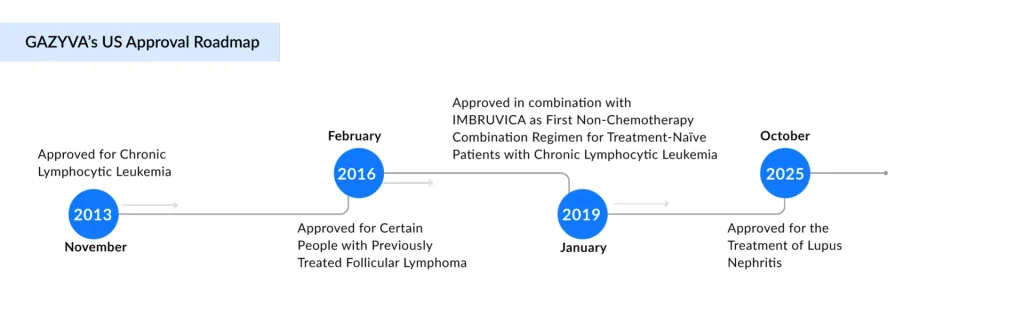
As the lupus treatment landscape undergoes major changes, Roche is entering the US lupus nephritis market with a fresh FDA approval for its long-standing blood cancer drug, GAZYVA. On October 20, 2025, Roche’s Genentech division announced that the FDA had approved GAZYVA (obinutuzumab) for use in adults with active lupus nephritis who are already receiving standard therapy. Initially approved in 2013 for chronic lymphocytic leukemia (CLL), GAZYVA also holds approvals for treating follicular lymphoma across several settings. The treatment involves four initial infusions in the first year, followed by maintenance doses given twice a year.
Lupus nephritis is a serious and potentially life-threatening complication of systemic lupus erythematosus (SLE), the most common form of lupus, that causes irreversible damage to the kidney’s filtering units, known as nephrons. Over time, many individuals with lupus nephritis experience a decline in kidney function, and despite advances in treatment, up to one-third eventually progress to end-stage kidney disease.
Lupus nephritis affects over 1.7 million people globally, with a disproportionate burden on women, particularly women of color and those of childbearing age, who tend to experience more severe forms of the disease. The total diagnosed prevalent cases of lupus nephritis in the 7MM were approximately 385,000 in 2024, and this number is projected to increase by 2034. The US contributed the largest prevalent population of Lupus nephritis, accounting for ~57% of the 7MM in 2024. Without proper treatment, as many as one in three patients may develop end-stage kidney disease, often necessitating dialysis or a kidney transplant.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- Roche’s Gazyva receives FDA Breakthrough designation for Lupus nephritis
- Emerging Lupus Nephritis Therapies: Addressing Unmet Needs through Innovation and Mechanistic Tar...
- Aelis Farma pockets $30M; Alcyone unveils $23M for AAV gene therapies; Progentec and GSK collabor...
- Systemic Lupus Erythematosus: An autoimmune disease
Roche’s GAZYVA, an anti-CD20 monoclonal antibody, offers a potential new approach. Originally developed for blood cancers, the therapy is being explored for its ability to protect the kidneys in lupus nephritis by curbing further damage and slowing or preventing progression to severe kidney failure. The rationale stems from the fact that lupus nephritis is primarily driven by B-cell activity. GAZYVA/GAZYVARO has received approval in 100 countries for the treatment of different haematological cancers. In the United States, the development and distribution of this product involves a partnership between Genentech and Biogen.
The GAZYVA approval is supported by positive findings from the phase II NOBILITY and phase III REGENCY trials. In the REGENCY study, nearly half of the participants (46.4%) treated with GAZYVA/GAZYVARO plus standard therapy achieved a complete renal response (CRR), compared to 33.1% of those receiving standard therapy alone. Treatment also led to significant improvements in complement levels, reductions in anti-dsDNA antibodies, corticosteroid use, and proteinuria — all indicators of better disease control. The safety profile of GAZYVA/GAZYVARO remained consistent with that previously observed in its haematology-oncology uses.
“Patients with lupus nephritis who reach a complete renal response have a higher chance of maintaining kidney function and slowing, or even preventing, the progression to end-stage kidney disease,” said Levi Garraway, MD, PhD, Roche’s Chief Medical Officer and Head of Global Product Development. “The FDA’s approval of GAZYVA/GAZYVARO represents a significant milestone toward establishing a potential new standard of care for lupus nephritis, offering clinicians the opportunity to provide patients with more effective disease management.”
Although Roche’s lupus nephritis drug showed positive results in renal response and disease activity measures, it fell short on secondary outcomes, including the average change in patients’ estimated glomerular filtration rates (eGFR), death or kidney-related events, and overall kidney responses. eGFR is a metric that assesses how effectively the kidneys filter waste and excess water from the blood to produce urine.
Currently, the lupus treatment market, spanning multiple indications, is primarily led by GSK’s biologic BENLYSTA, a B-lymphocyte stimulator (BlyS)–specific inhibitor that was first approved by the FDA in 2011. More recently, in 2021, AstraZeneca received FDA approval for SAPHNELO to treat moderate to severe systemic lupus erythematosus.
“Lupus nephritis is a serious, potentially life-threatening condition that can profoundly affect daily life, causing persistent pain, fatigue, and ongoing concern over kidney health,” stated Louise Vetter, President and CEO of the Lupus Foundation of America. “The FDA’s approval of GAZYVA/GAZYVARO brings new hope to individuals with lupus nephritis and their families, offering an important treatment option that may help prevent long-term complications, including kidney failure.”
Physicians have also used Roche’s RITUXAN off-label for lupus nephritis treatment for some time. Roche had previously attempted to gain FDA approval for the CD20 antibody for lupus, but was unsuccessful after Rituxan failed a phase III trial. Meanwhile, GAZYVA has steadily grown through its oncology portfolio, achieving 16% growth to 910 million Swiss francs (~$1.15 billion) in 2024, according to Roche’s latest annual report.
The FDA granted GAZYVA/GAZYVARO Breakthrough Therapy Designation in 2019 based on NOBILITY phase II data. The FDA approval comes just days after the European Medicines Agency’s Committee for Medicinal Products for Human Use issued a positive recommendation for GAZYVA, in combination with mycophenolate mofetil, for lupus nephritis. The treatment will now await final regulatory approval from the European Commission.
GAZYVA’s entry into the US lupus nephritis landscape is likely to create a competitive shift, particularly in the biologics segment. While BENLYSTA remains the standard-of-care for many patients, GAZYVA’s differentiated mechanism of action as an anti-CD20 therapy provides clinicians with an alternative for patients who have an inadequate response to existing therapies. Analysts note that GAZYVA’s semiannual dosing regimen may also improve patient adherence compared to more frequent dosing schedules, potentially enhancing real-world effectiveness and long-term kidney outcomes. With the US representing the largest lupus nephritis patient population in the 7MM, Roche is well-positioned to capture a significant share. However, market uptake will depend on physician familiarity, payor coverage, and comparative efficacy data.
DelveInsight projects GAZYVA sales to reach USD 400 million by 2034, reflecting both the sizable addressable population and unmet needs in lupus nephritis. Analysts caution, however, that GAZYVA’s modest performance on secondary endpoints, such as eGFR stabilization, may temper expectations among some clinicians and payors. Nevertheless, the therapy’s approval signals Roche’s commitment to expanding its immunology portfolio beyond oncology, while reinforcing the broader trend of repurposing established hematology drugs for autoimmune indications. Strategic partnerships, ongoing post-marketing studies, and potential expansion into pediatric lupus nephritis and other kidney disorders could further bolster long-term growth prospects.
Beyond lupus nephritis, GAZYVA/GAZYVARO is also being studied in systemic lupus erythematosus, membranous nephropathy, idiopathic nephrotic syndrome, and pediatric lupus nephritis. Roche continues to advance a broad pipeline targeting key immune pathways involved in both rare and common kidney diseases.

Downloads
Article in PDF
Recent Articles
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- FDA Approves Genentech’s GAZYVA for Lupus Nephritis; Glaukos’ EPIOXA Wins FDA Approval for Kerato...
- 7 Late-Stage SLE Drugs Expected to Enter Therapeutic Domain
- HUTCHMED’s NDA Submission to FDA for Fruquintinib; Cytokinetics to Discontinue ALS Drug Candidate...
- Aelis Farma pockets $30M; Alcyone unveils $23M for AAV gene therapies; Progentec and GSK collabor...



