Peanut Allergy Treatment Space: Expanding Horizons Beyond Traditional Immunotherapy
Oct 20, 2025
Table of Contents
Peanut allergy is among the most common and serious types of food allergy, with prevalence increasing over the past few decades, particularly among younger populations. It usually first appears in childhood, with symptoms emerging as early as 4 months of age and most often within the first two years of life. Around 20% of children eventually outgrow their peanut allergy and can tolerate peanuts later without adverse reactions. In the United States, peanut allergy is one of the nine most prevalent food allergies, affecting roughly 1–2% of the population.
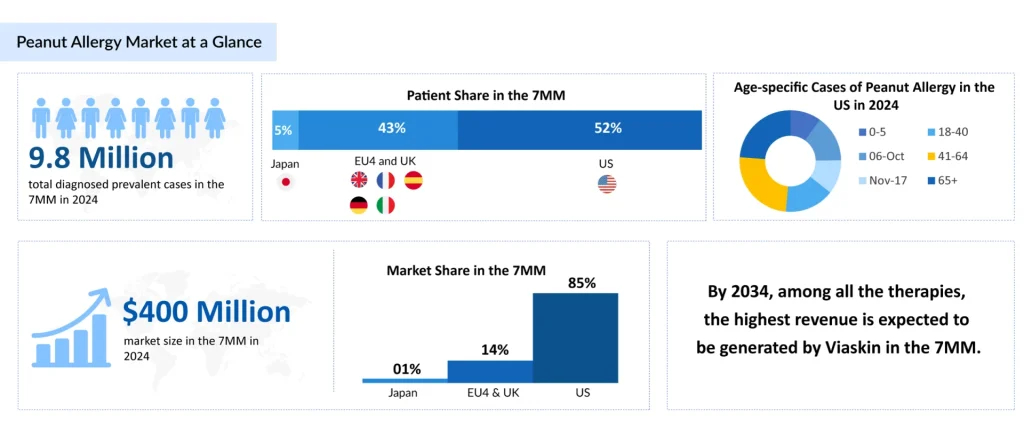
According to DelveInsight’s 2024 assessment, there were approximately 9.8 million diagnosed cases of peanut allergy across the leading markets. Mild-to-moderate peanut allergy represented the largest burden, impacting nearly 4.9 million individuals in 2024. Trend analyses indicate a steady increase in cases over the study period from 2020 to 2034.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Revolutionizing the Food Allergy Treatment: The Impact of Xolair’s Approval
- What are the Top 9 Most Common Food Allergies and How Pharma Companies are Tackling the Crisis?
- First-ever Peanut allergy treatment; Dinutuximab misses primary endpoint; Insmed’s shares S...
- Notizia
- Peanut Allergy Market Outlook: The Market Size Growth Sees Major Thrusts
Currently, the primary approach to managing peanut allergy involves strict avoidance of peanuts and the use of an Adrenaline Auto-injector (AAI) to treat allergic reactions when they occur.
PALFORZIA and XOLAIR: Established Peanut Allergy Treatments
At present, Stallergenes Greer’s PALFORZIA and Roche and Novartis’ XOLAIR remain the only approved therapies offering meaningful treatment options for patients with peanut allergies and their healthcare providers. PALFORZIA became the first FDA-approved drug for peanut allergy in children in January 2020, with initial projections estimating sales of USD 1.28 billion by 2024. However, its market performance fell short of expectations, partly due to challenges from the COVID-19 pandemic and the drug’s complex administration requirements, which necessitated frequent visits to healthcare providers.
On the other hand, XOLAIR is an FDA-approved medication designed to reduce allergic reactions in individuals with one or more food allergies. It is administered as a subcutaneous injection, either by a healthcare professional or by self-injection at home after an initial supervised dose. In February 2024, the FDA expanded XOLAIR’s approval to include the reduction of allergic reactions, including anaphylaxis, from accidental exposure to one or more foods in both adults and children aged 1 year and older with IgE-mediated food allergy.
In March 2025, the FDA approved OMLYCLO (omalizumab-igec) as the first biosimilar designated interchangeable with XOLAIR for treating IgE-mediated food allergy. Meanwhile, ongoing clinical trials in SLIT, OIT, and EPIT immunotherapies show promising results, with OIT currently standing as the only FDA-approved immunotherapy. Overall, the peanut allergy treatment market is increasingly moving toward immunotherapies and biologics.
Peanut Allergy Drugs Under Development
The peanut allergy clinical trial landscape includes several drugs in the development stage that are expected to be approved in the near future. The emerging landscape holds therapeutic alternatives for treatment, including Viaskin (DBV Technologies), PVX108 (Aravax), Remibrutinib (Novartis), SLIT tablet (ALK-Abello), and others.
DBV Technologies’ Viaskin Peanut is an epicutaneous immunotherapy (EPIT) that delivers small amounts of peanut protein via a wearable patch to promote desensitization. The therapy has received both Fast Track and Breakthrough Therapy designations from the US FDA. It is currently being tested in a Phase III clinical trial involving children aged 4–7 with peanut allergies. DBV Technologies expects to release top-line results from the VITESSE trial (NCT05741476) in Q4 2025.
Aravax’s PVX108 is an advanced immunotherapy aimed at retraining the immune system. It uses engineered peptides to target T cells and potentially reverse allergic responses selectively. The therapy is currently in a Phase II clinical trial for children and adolescents with peanut allergies.
Novartis’ Remibrutinib is a highly selective, potent, oral covalent Bruton’s Tyrosine Kinase (BTK) inhibitor under development for autoimmune conditions, including chronic spontaneous urticaria, myasthenia gravis, and multiple sclerosis, as well as food allergies and hidradenitis suppurativa. It is presently being evaluated in a Phase II clinical trial for adults with peanut allergies.
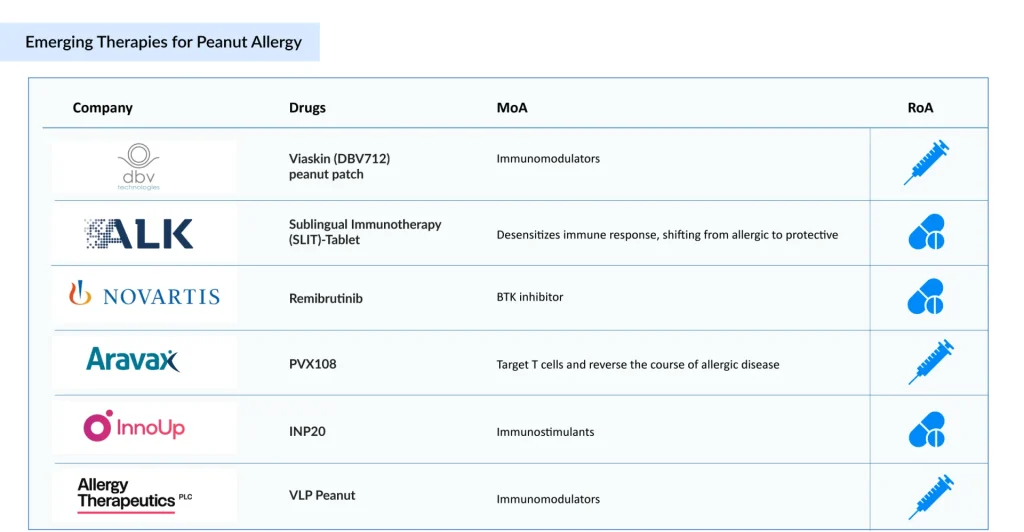
The anticipated launch of these emerging peanut allergy therapies are poised to transform the treatment market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the peanut allergy treatment market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Recent Developments in Peanut Allergy Treatment Space
- In March 2025, DBV Technologies reached an understanding with the US FDA based on written replies to its Type D IND meeting request. The FDA concurred with DBV’s proposal that safety exposure data from the VITESSE Phase III study of the Viaskin Peanut Patch in children aged 4–7 years would be sufficient to support BLA filing for this age group, thereby accelerating the anticipated timeline for BLA submission to the first half of 2026.
- In March 2025, the US FDA approved OMLYCLO (omalizumab-igec) as the first interchangeable biosimilar for reference XOLAIR and the first respiratory biosimilar for the treatment of moderate-to-severe persistent asthma, Chronic Rhinosinusitis with Nasal Polyps (CRSwNP), IgE-mediated food allergy, and Chronic Spontaneous Urticaria (CSU).
- In March 2025, DBV Technologies announced a financing of up to USD 306.9 million (EUR 284.5 million), comprising gross proceeds of USD 125.5 million (EUR 116.3 million) to be received upon closing and up to an additional USD 181.4 million (EUR 168.2 million) in gross proceeds contingent upon the full exercise of warrants, subject to the satisfaction of specified conditions.
- In February 2025, Stallergenes Greer launched PALFORZIA in the US to treat toddler patients, ages 1–3 years, with a confirmed diagnosis of peanut allergy. The FDA approved the expanded indication for toddler patients (ages 1–3 years) in July 2024 based on the data from the Phase III POSEIDON (Peanut Oral Immunotherapy Study of Early Intervention for Desensitization) study that was published in the New England Journal of Medicine Evidence in 2023.
- In January 2025, the European Commission approved the extension of the indication of PALFORZIA for the treatment of toddlers (ages 1–3) with a confirmed diagnosis of peanut allergy. The marketing authorization covered all 27 European member states and the three European Economic Area states (Iceland, Liechtenstein, and Norway).
What Lies Ahead in Peanut Allergy Treatment?
The landscape of peanut allergy treatment is rapidly evolving, moving beyond traditional avoidance strategies and emergency interventions. The total peanut allergy market size in the 7MM was estimated to be nearly USD 400 million in 2024, and it is expected to grow positively at a CAGR of 16% by 2034. This change is primarily attributed to the adoption of recently approved therapies, the emergence of new treatments, an increase in diagnoses, and rising costs.
One of the most promising approaches on the horizon is oral and epicutaneous immunotherapy, which aims to desensitize patients by gradually introducing controlled amounts of peanut protein. Epicutaneous immunotherapy, delivered via a wearable patch, offers a less invasive alternative to oral routes, potentially reducing the risk of severe allergic reactions while still building tolerance. Clinical trials are showing encouraging results, particularly in younger children, where early intervention could reshape immune responses and improve quality of life.
Looking forward, research is also exploring biologic therapies, such as monoclonal antibodies, that target the underlying immune mechanisms driving allergic reactions. These therapies could complement or even replace existing desensitization methods, offering personalized treatment options based on an individual’s risk profile and immune response.
Additionally, combination strategies, pairing biologics with immunotherapy, are under investigation, aiming to enhance safety and efficacy while shortening treatment duration. As our understanding of peanut allergy deepens, the future may hold not only better symptom management but also the possibility of long-term tolerance, transforming the outlook for millions of individuals affected by this condition.

Downloads
Article in PDF
Recent Articles
- What are the Top 9 Most Common Food Allergies and How Pharma Companies are Tackling the Crisis?
- First-ever Peanut allergy treatment; Dinutuximab misses primary endpoint; Insmed’s shares S...
- Revolutionizing the Food Allergy Treatment: The Impact of Xolair’s Approval
- Notizia
- Zydus starts Phase II COVID-19 vaccine trial; Taysha raises $95M; FDA declines DBV’s peanut...



