Rare Disease
Mar 01, 2019
Cost ineffective Orphan Drugs limit the access
Rare diseases are mostly serious, chronic and life-threatening, associated with both psychological as well as financial burdens. In addition, only a few of them have effective drug treatment available. The European Union (EU) definition of a rare disease is one that affects fewer than 5 in 10,000 people. These affe...
Read More...
Feb 28, 2019
Rare Disease Day
A rare disease is any disease that affects a small percentage of the population. In some parts of the world, an orphan disease is a rare disease whose rarity means there is a lack of a market large enough to gain support and resources for discovering treatments for it, except by the government g...
Read More...
Sep 04, 2018
Orphan Drug Act and Its Criticism
The Orphan Drug Act of 1983 was created to encourage pharmaceutical companies to develop drugs for so-called rare or ultra-rare diseases. The National Institutes of Health (NIH) defines rare diseases as afflicting as fewer than 200,000 individuals. Generally speaking, it has worked. Many companies are willing to inv...
Read More...
May 22, 2018
Celiac vaccine trial; New discovery in Nicotine; Nabriva lines up FDA filing; Rare Disease drug development accelerating
ImmusanT upgrading the management ahead of phase 2 celiac vaccine trial ImmusanT has upgraded its senior staff by appointing Daiichi Sankyo’s SVP for internal medicine therapeutics, Ken Truitt, M.D., as its new chief medical officer. The management is also boosted with new VPs in program management and clinical oper...
Read More...
Nov 06, 2017
Wiskott – Aldrich syndrome – a rare X-linked disorder
Wiskott - Aldrich syndrome (also known as WAS) is a rare X-linked recessive genetic disorder that affects both T-lymphocytes and B-lymphocytes. It occurs due to the mutations caused in the WASp gene and is mainly characterized by low platelet count (microthrombocytopenia), immune deficiency, and eczema. Being an X-...
Read More...
Sep 12, 2017
Idera’s Phase I Data; DelMar Initiates Trial; Novartis’ study; Humira gets EC approval
Idera Pharmaceuticals Present Positive Phase I Data for their Drug candidate IMO-2125 Idera Pharmaceuticals announced final results from the dose-selection phase of an ongoing Phase 1/2 trial investigating IMO-2125 in combination with ipilimumab (Yervoy). The combination dose-selection phase included 18 patients, al...
Read More...
Jun 27, 2017
Takeda knocks Velcade; FDA nod awaited; Novartis gets approval; Astellas UK dodges; Haegarda set for launch
With patent loss looming, Takeda knocks proposed Velcade copycats in FDA petition Takeda’s multiple myeloma blockbuster Velcade is quickly running out of patent protection, but the drug maker has filed a citizen petition it hopes can stave off competition for a little while longer. The Japanese pharma giant asked th...
Read More...
Dec 13, 2016
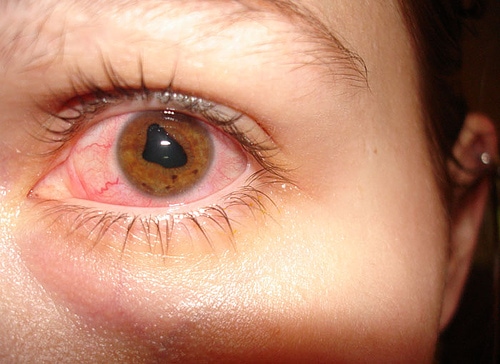
Anterior Uveitis: A Vision-threatening condition
Anterior uveitis is a set of conditions defined by intraocular inflammation and is believed to be the cause of up to 10% of legal blindness in the United States, or approximately 30,000 new cases of blindness per year. Anterior uveitis is an intraocular inflammation of the uveal structures. The uvea is situated ante...
Read More...
Sep 23, 2016
FDA Approves Sarepta’s Muscular Dystrophy Drug after Months of Debate
The Food and Drug Administration approved the recent controversial drug to treat Duchenne muscular Dystrophy, which is a rare disease that confines boys to wheelchairs and condemns them to an early death. The decision was made after months of debate between the Agency and Sarepta Therapeutics, regarding the evidence...
Read More...
Oct 13, 2015
Rising of Orphan Drug Development
Introduction: Any disease that affects less than 200,000 patients or 1 in 1500 is termed as a rare disease. There are around 5000 to 8000 rare disease reported, out which only 10% have cure. According to the European Organization for rare disease, 80% of rare diseases are genetic in nature. Some of the rare disease...
Read More...





-Agonist.png)

