T-cell Immunoglobulin and ITIM domain (TIGIT) Inhibitor: An Insight into the Pipeline Development Activities, Major Collaborations, and Advancements
Dec 06, 2021
Table of Contents
T-cell immunoreceptor with Ig and ITIM domains (TIGIT) is a relatively new immunological checkpoint that has been studied as a potential immunotherapeutic target. TIGIT is a transmembrane glycoprotein receptor containing an Ig-like V-type domain in its cytoplasmic domain and an ITIM in its extracellular domain. It’s found in activated and memory T cells, as well as NK cells and Tregs. CD155 (PVR: poliovirus receptor) and CD112 (PVRL2, nectin-2) bind to each other on APCs, preventing maturation and conferring a tolerogenic phenotype. Numerous clinical trials on TIGIT-blockade in cancer have been initiated, predominantly combination treatments.
TIGIT Structure
TIGIT is a member of a growing family of PVR-like proteins. It was independently discovered in 2009 by three groups through genome-wide analysis aiming to identify proteins containing domain structures typical for immunomodulatory receptors. TIGIT consists of one extracellular immunoglobulin variable domain, a type I transmembrane domain, and a short intracellular domain with one immunoreceptor tyrosine-based inhibitory motif (ITIM) and one immunoglobulin tyrosine tail (ITT)-like motif.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Next Chapter in NSCLC Treatment Space: Recent Discoveries and Innovations
- Coherus and Junshi’s Loqtorzi: First-ever Chinese PD-1 Inhibitor and Nasopharyngeal Carcinoma Tre...
- AZ, Daiichi inks an oncology deal; Moderna begins Phase III trial of its COVID-19 vaccine; No goo...
- Unlocking New Avenues in KRAS-Driven Cancer Research Beyond G12C
- Evotec, Takeda Deal; AZ COVID Vaccine US Trials; Promega’s Recognition; Roche’s Tecentriq R...
TIGIT Expression
TIGIT is expressed on CD4+ T cells, CD8+ T cells, and Tregs in both mice and humans. TIGIT expression is normally low in naive cells, but T and NK cells have been shown to up-regulate TIGIT after activation. As a result, Tregs, memory and activated T cells, and NK cells have the highest TIGIT expression in naive mice and healthy individuals.
Cells Expressing TIGIT Receptors and Ligands
TIGIT’s Ligands
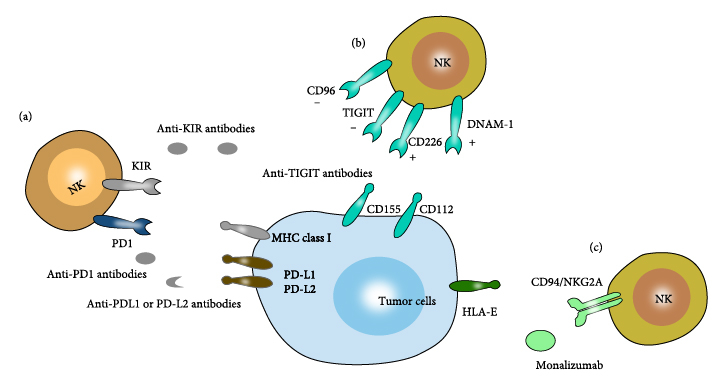
TIGIT has three ligands: CD155, CD112, and CD113, which are all members of the nectin and NECL family of molecules. This family includes cell surface molecules that mediate cell adhesion, cell polarity, and tissue organisation, as well as herpes and poliovirus receptors. TIGIT’s major ligand is CD155, which is found in both humans and mice. TIGIT and CD155 form homodimers and heterotetramers after ligand-receptor contact, according to crystal structure studies. TIGIT has a lower affinity for CD112 and CD113 than CD155.
TIGIT Mechanisms of Action
TIGIT-mediated inhibitor of effector T cells and NK cells has been linked to a variety of processes. TIGIT can function either cell-extrinsically as a CD155 ligand or cell-intrinsically by interfering with DNAM-1 co-stimulation or directly providing inhibitory signals to effector cells. It’s unknown whether all of these processes are active in every TIGIT-expressing cell or if the TIGIT method of action varies between CD4+ T cells, CD8+ T cells, and NK cells. TIGIT also improves Treg suppressive capabilities when expressed on Tregs, potentially inhibiting a wide range of immune cells.
Cell-extrinsic Mechanism
Based on the discovery that neither TIGIT-specific small interfering RNA (siRNA) nor anti-TIGIT mAbs impacted human memory CD4+ T cell responses to antiCD3 stimulation, early investigations suggested a cell-extrinsic mechanism. Anti-TIGIT mAbs, on the other hand, boosted T cell proliferation and IFN- production when T cells were cultivated with autologous CD11c+ DCs. Antigen-presenting cells (APCs) such as DCs are essential for priming T cell responses. The sort of DCs used and their maturity stage determine the quality of the T cell response elicited. TIGIT’s interaction with CD155 regulated DC cytokine production, according to Yu et al. CD155 signalling in human monocyte-derived DCs boosted IL-10 secretion while decreasing proinflammatory cytokine IL-12 secretion after TIGIT ligation. TIGIT–CD155 interactions appear to promote tolerogenic DCs that suppress T cell responses, according to these findings.
Cell-intrinsic Mechanisms
TIGIT suppresses T cell activities in a cell-intrinsic manner, in addition to its activity as a regulator of DCs and macrophages. In the absence of APCs, agonistic anti-TIGIT mAbs have been demonstrated to suppress anti-CD3/anti-CD28 mAb-mediated human and mouse T cell proliferation and cytokine generation. Furthermore, melanoma cells expressing a shortened variant of CD155 reduced CD8+ T cell IFN- production comparable to cells expressing wild-type CD155, according to a recent study. TIGIT–CD155 contact can decrease T cell functions without CD155 downstream signalling, indicating that TIGIT–CD155 interaction can inhibit T cell functions without CD155 downstream signalling. Given TIGIT’s strong affinity for CD155, the cell-intrinsic mechanism of action was speculated to be TIGIT inhibiting T cells by out-competing DNAM-1 for CD155 binding.
The Target for Cancer Immunotherapy
Even before the discovery of TIGIT, its ligands were known to be upregulated on the surface tumour cell surface. Expression of the nectin family of proteins and their role in cell adhesion and survival was reported in tumors from the epithelial origin, such as non-small cell lung cancer, colon cancer, and metastatic neuroblastoma, and also tumors from the hematopoietic origin, such as myeloid leukaemia. High expression of CD155 was shown to be an independent prognostic marker and predictor of poor clinical outcomes in breast cancer patients. The recently discovered ligand for TIGIT, nectin 4, was shown to be overexpressed in breast, bladder, lung, and pancreatic cancers. On the other hand, TIGIT expression was also reported to be upregulated on lymphocytes in the tumour microenvironment. Studies showed TIGIT expression on CD8+, CD4+ T cells, and NK cells paralleled to that of PD-1 in the hepatocellular, lung, and colorectal cancers and in Hodgkin’s lymphoma. The potential of targeting TIGIT was shown using in-vivo mouse models for cancers and chronic viral infection. Researchers showed that blockade of TIGIT along with PD-L1 enhanced CD8+ T-cell effector function and tumour and viral clearance. Studies in tumour-bearing Tigit+/+ and Tigit−/− mice demonstrated increased TIGIT expression on tumour-infiltrating lymphocytes and lack of TIGIT in Tregs to be critical for immune suppression in the tumour microenvironment. Administration of monoclonal antibodies against TIGIT was shown to increase the survival rate in mouse models for ovarian cancer. A study found that the anti-TIGIT antibody treatment reduced CD4+ Tregs but did not affect the proportion of CD4+ T-helper cells, CD8+ T cells, or NK cells.
Collaborations Assessment- Licensing / Partnering / Funding
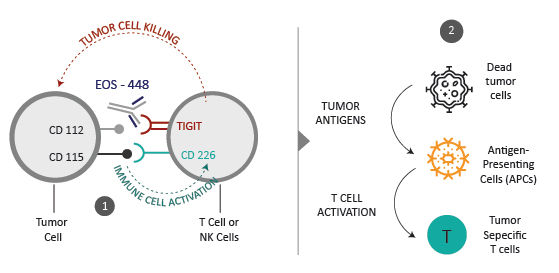
Recent research has identified TIGIT as a promising emerging immune checkpoint target. TIGIT indirectly suppresses T-cell activation and is expressed on multiple types of immune cells, including regulatory T (Treg) cells, activated T cells, and natural killer (NK) cells. TIGIT is upregulated in T cells and NK cells after ligand binding, inhibiting these cells’ ability to kill tumour cells. Increased TIGIT expression is also associated with advanced disease and poor survival outcomes. Additionally, increased TIGIT expression after treatment is associated with disease recurrence. TIGIT and its coreceptors, including CD226 and CD96, interact with a group of ligands expressed on tumour cells: nectins and nectin-like molecules, including poliovirus receptor (PVR), the main TIGIT ligand. PVR ligands binding to TIGIT suppresses the antitumor immune response of the TIGIT-expressing cell; in contrast, ligand binding to CD226 triggers immune cell activation. Thus, TIGIT and CD226 oppose each other to regulate tumour immunity. Anti-TIGIT therapy is presumed to invigorate immune function and enhance the activity of T cells and NK cells. ( Explore more about NK cells in cancer)
Treatment Landscape Through the Lens of TIGIT Inhibitors
One avenue of investigation in recent years has been immune-oncology, which encourages the human body to make use of its natural defences to combat cancer. The activation of immune cells against tumors is seen to be an extremely promising approach, as it allows the efficient eradication of existing tumour cells through the generation of long-term protective immune memory. Moreover, unlike common oncologic treatments, immune activation is associated with targeted effects and lower toxicity and may lead to extremely long recurrence-free events due to the generation of this protective memory.
There have been quite exciting developments in the treatment development landscape involving the TIGIT Inhibitor-based therapies. The drugs which are under development are being investigated in trials both as monotherapy as well as in combination with some other drugs; however, the majority of the TIGIT inhibitor drugs are being developed as combination therapy.

Some of the notable ones include therapies being developed by Bristol Myers Squibb, Roche, GlaxoSmithKline and iTeos Therapeutics, Gilead, and AstraZeneca, to name a few.
The development milestones have been discussed below, which highlight the activity around this target, showcasing great potential in the cancer immunotherapy area.
Just some months before, in January 2021, Roche (Genentech) announced that tiragolumab, novel cancer immunotherapy designed to bind to TIGIT, received Breakthrough Therapy Designation (BTD) by the US Food and Drug Administration (FDA), in combination with Tecentriq (atezolizumab) for metastatic non-small cell lung cancer (NSCLC) treatment as a first-line in people whose tumors have high PD-L1 expression with no EGFR or ALK genomic tumour aberrations. Tiragolumab is the first anti-TIGIT molecule to be granted BTD from the FDA, and the designation is based on randomized data from the phase II CITYSCAPE trial. CITYSCAPE provides the first evidence that targeting both immune inhibitory receptors, TIGIT and PD-L1, may enhance anti-tumour activity by potentially amplifying the immune response.
Domvanalimab, an advanced anti-TIGIT candidate, is an Fc-silent investigational monoclonal antibody that blocks TIGIT and has demonstrated complete receptor coverage on all TIGIT-expressing peripheral leukocytes. This investigational medicine is designed to treat solid tumors because the silent Fc domain is believed to eliminate the potential for depletion of TIGIT-bearing immune cells, which are critical for anti-tumour immune activity. Domvanalimab is currently being evaluated in an ongoing Phase III registrational study in combination with zimberelimab, an anti-PD-1 monoclonal antibody in first-line locally advanced or metastatic, PD-L1>50% non-small cell lung cancer.

Most recently, in August 2021, Compugen announced that a bispecific antibody of AstraZeneca derived from COM902, Compugen’s high-affinity anti-TIGIT antibody, will advance into clinical development. AstraZeneca plans to initiate a Phase I/II study evaluating AZD2936, a TIGIT/PD-1 bispecific antibody, in patients with advanced or metastatic non-small cell lung cancer. Some other notable TIGIT inhibitors being developed include Ociperlimab (BGB-A1217) and MK-7684A. Ociperlimab is an investigational humanized monoclonal antibody designed to bind to TIGIT with high specificity and affinity. Ociperlimab is one of the most advanced anti-TIGIT antibodies in development with an intact IgG Fc binding region for optimal antibody-mediated anti-tumour activity. MK-7684A is a formulation of Vibostolimab/pembrolizumab which is being developed by Merck and is currently in Phase III stage of development for Non-small cell lung cancer treatment and other indications in lower phases.
The Pipeline Scenario of TIGIT Inhibitors
What’s cooking in the Lab?
Apart from the clinical assets in development, there have been some interesting advancements from the preclinical/discovery aspect as well. Zhang et al. reported that early treatment with anti-TIGIT mAbs (i.e. started before the tumour was established) delayed the growth of CT26 subcutaneous tumors and methylcholanthrene (MCA) – induced fibrocarcinomas. The same group showed that anti-TIGIT mAbs protected mice against 4T1 or B16 experimental metastasis. Interestingly, Zhang et al. proposed that NK cells were involved in the protection observed following TIGIT-blockade in all these different models. Similarly, we found that blocking TIGIT significantly decreased tumour burden in an aggressive mouse myeloma model (Vk12653) and increased survival in two additional mouse myeloma models, Vk12598 and 5TGM1.
“TIGIT inhibits T-cell activation,” Vijay K. Kuchroo, DVM, PhD, the Samuel L. Wasserstrom Professor of Neurology at Harvard Medical School and a senior scientist in neurology at Brigham and Women’s Hospital in Boston, Massachusetts, who has studied TIGIT for the past 15 years, said in an interview with Targeted Therapies in Oncology. “Our data suggest that [inhibiting] it cooperates well with PD-1 blockade.” Tumors use the inhibitory TIGIT pathway to avoid attack by the immune system. Thus, therapeutic agents that inhibit TIGIT signalling by, for example, disrupting ligand binding promote the ability of T cells to proliferate and attack tumour cells.
Summary of Key Findings
Conclusion
TIGIT has emerged as an important immune checkpoint capable of inhibiting each step of the cancer immunity circle due to its broad expression on lymphocytes. Tumour antigen release by NK cells may be prevented by TIGIT, impairing T cell priming by DCs or inhibiting cancer cell killing by CD8+ T cells. When combined with other immunotherapies, TIGIT targeting is likely to be the most efficient.
A significant number of preclinical studies indicated that TIGIT would constitute a suitable target for cancer patients and the results of six ongoing clinical trials are eagerly awaited. In pre-clinical studies, targeting TIGIT together with the PD-1/PD-L1 pathway demonstrated superior tumour suppression compared to monotherapy. TIGIT has also been found to regulate antiviral responses and perhaps impair immune control to human immunodeficiency virus. Combination therapy using anti-TIGIT and anti-PD-1 antibodies further conferred a greater survival benefit in all anti-TIGIT treatment schedules, implying a synergistic mechanism following interruption of the two inhibitory checkpoints. This improvement in therapeutic efficacy following dual blockade of the TIGIT and PD-1 pathways has been demonstrated in several murine models.
The design of optimized combination strategies for TIGIT blockade in cancer patients is being facilitated with the increasing understanding of TIGIT-mediated regulation of immune responses and will also help to treat other chronic diseases with the development of TIGIT- targeting therapies. Thus, TIGIT inhibitors are creating great inroads in the Immuno-oncology space by providing better opportunities, as late-stage clinical research is evaluating this inhibitor in combination with PD/L-1 inhibitors in high PD-L1, refractory PD-1, and adjuvant settings, especially in NSCLC patients.
Downloads
Article in PDF
Recent Articles
- European Lung Cancer Congress (ELCC) 2025 Recap: Advances in Lung Cancer Research and Treatment
- C4X Discovery and AstraZeneca Signs Deal; FDA Rejects Spectrum’s Poziotinib; Orphan Drug Designat...
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
- Post WCLC 2024 Insights: Recent Advancements in Thoracic Malignancies
- Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Sclet...



