Dizal’s ZEGFROVY Approval Heating Up EGFR NSCLC Drug Rivalry with J&J
Jul 21, 2025
Eight years after its founding, partly backed by AstraZeneca, Dizal Pharmaceuticals has secured its first FDA approval, receiving accelerated clearance for ZEGFROVY (sunvozertinib)—now the only FDA-approved oral therapy for a rare subset of non-small cell lung cancer (NSCLC).
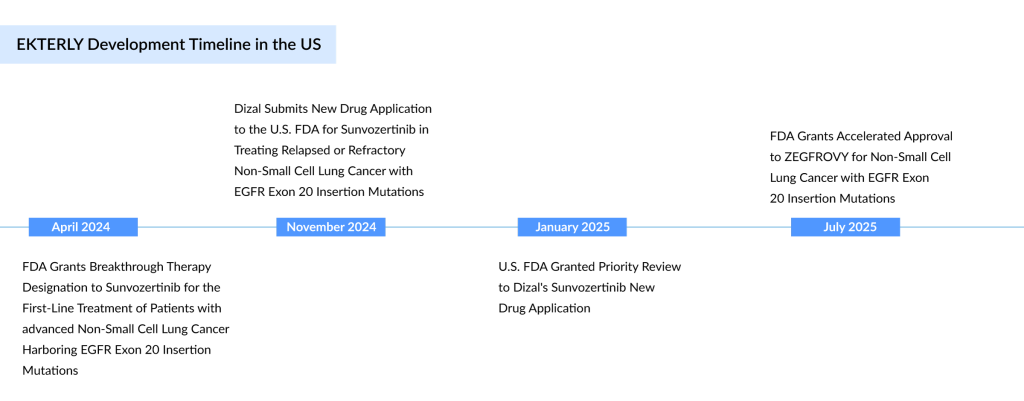
The treatment is indicated for U.S. patients with EGFR exon 20 insertion mutations whose cancer has progressed after platinum-based chemotherapy. This milestone follows 16 months after the FDA approved Johnson & Johnson’s RYBREVANT, an infused EGFR-MET bispecific antibody, for first-line treatment in combination with chemotherapy. Unlike Rybrevant, ZEGFROVY is a first-in-class, selective EGFR tyrosine kinase inhibitor (TKI). ZEGFROVY is an oral, irreversible EGFR inhibitor with a unique molecular design, targeting a broad range of EGFR mutations while maintaining selectivity for wild-type EGFR. It previously received accelerated approval in China in August 2023. This FDA decision follows the drug’s receipt of Breakthrough Therapy Designation and Priority Review from both the US FDA and China’s NMPA (Center for Drug Evaluation).
“We are delighted to introduce ZEGFROVY, the first oral therapy of its kind, providing a more effective treatment option with improved safety and convenient administration for NSCLC patients with EGFR exon20ins,” said Dr. Xiaolin Zhang, CEO of Dizal. “This accelerated approval represents a major milestone and reaffirms our dedication to delivering innovative medicines that address critical unmet needs for patients worldwide.”
Downloads
Click Here To Get the Article in PDF
Recent Articles
- STAT INHIBITORS- Highly active Pipeline
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
- Merck and Moderna Initiate Study to Evaluate V940; FDA Approves Vertex and CRISPR Therapeutics’ C...
- Checkpoint Inhibitors: A Potential Approach in the Fight Against Refractory Cancer
- T-cell Immunoglobulin and ITIM domain (TIGIT) Inhibitor: An Insight into the Pipeline Development...
Dizal is aiming to succeed where a previous oral EGFR inhibitor fell short. Takeda’s EXKIVITY, which received accelerated FDA approval for second-line treatment in 2021, was pulled from the US market two years later after failing to outperform chemotherapy in a confirmatory study.
The FDA’s approval of ZEGFROVY is based on results from WU-KONG1 Part B (WU-KONG1B), a multinational pivotal trial evaluating ZEGFROVY in patients with relapsed or refractory NSCLC harboring EGFR exon 20 insertions. The data were presented at the 2024 ASCO Annual Meeting and recently published in the Journal of Clinical Oncology.
The WU-KONG1B trial demonstrated tumor shrinkage in 46% of patients and a median response duration of 11.1 months. For comparison, Rybrevant received its accelerated approval in 2021 with a 40% objective response rate (ORR) and the same median duration of response, while Exkivity’s supporting trial reported a 28% ORR.
“As the first and only approved targeted oral therapy for EGFR exon20ins NSCLC, ZEGFROVY has transformed the treatment landscape in an area that has long lacked convenient and effective options,” said Pasi A. Jänne, MD, PhD, of Dana-Farber Cancer Institute, Harvard Medical School, and lead principal investigator of WU-KONG1B. “Data from the WU-KONG1B study highlight ZEGFROVY’s strong therapeutic benefits and consistent efficacy across both Asian and non-Asian patients. It’s simple, once-daily oral regimen significantly enhances ease of administration and patient adherence—an increasingly vital factor as lung cancer care evolves toward chronic disease management. The U.S. approval of ZEGFROVY® represents a major scientific milestone and a critical step forward in meeting the long-unmet needs of this underserved patient population.”
“ZEGFROVY has shown significant therapeutic benefits in treating EGFR exon20ins NSCLC, as demonstrated in a robust multinational clinical trial. With its strong antitumor efficacy, manageable safety profile, and convenient oral dosing, it stands out as an excellent option for clinical use,” said Prof. James Chih-Hsin Yang, MD, PhD, from the National Taiwan University Cancer Center Hospital and Co-lead Principal Investigator of WU-KONG1B. “Its approval in key global markets brings new hope to patients and underscores our dedication to patient-focused research and the ongoing advancement of precision medicine in lung cancer.”
Notably, WU-KONG1B also showed that ZEGFROVY had anti-tumor activity regardless of prior Rybrevant treatment. The study included patients from Asia, Europe, North America, and South America. The FDA has also granted approval for Thermo Fisher Scientific’s Oncomine Dx Express Test as a next-generation sequencing (NGS) companion diagnostic (CDx) for ZEGFROVY, enabling the identification of NSCLC patients with EGFR Exon20 insertions.
NGS is a key technology for cancer genomic profiling, allowing rapid and accurate detection of tumor DNA mutations. When paired with the Ion Torrent Genexus Dx System, the test can deliver results in as little as 24 hours, supporting timely treatment decisions for patients with EGFR exon20ins NSCLC.
Meanwhile, Dizal has completed patient enrollment in its global phase III WU-KONG28 trial, comparing ZEGFROVY to platinum-based doublet chemotherapy in treatment-naïve NSCLC patients harboring EGFR exon20ins mutations across 16 countries and regions. Data presented at the 2023 European Society for Medical Oncology (ESMO) Annual Meeting showed that ZEGFROVY, as a single oral agent, achieved a confirmed objective response rate (ORR) of 78.6% and a median progression-free survival (mPFS) of 12.4 months in the first-line setting. With its robust antitumor efficacy and favorable safety profile, ZEGFROVY exhibits strong potential as a leading first-line therapy for EGFR exon20ins NSCLC patients.
The approval of ZEGFROVY marks a significant milestone for Dizal; however, the company is also facing growing competition from other pharmaceutical firms developing therapies for EGFR-mutated NSCLC. Companies such as Cullian Therapeutics/Taiho (Zipalertinib), ArriVent BioPharma (Firmonertinib), Avistone Biotechnology (PLB1004), and Pierre Fabre Laboratories (PFL-721 and PFL-241), among others, are currently working on their lead therapies for EGFR exon20ins NSCLC patients.
Zipalertinib (CLN-081/TAS6417) is an orally administered covalent inhibitor targeting mutant EGFR. A global Phase III trial (REZILIENT3; NCT05973773) initiated in August 2023 is evaluating zipalertinib in combination with chemotherapy as a potential first-line therapy for adults with previously untreated, locally advanced, or metastatic NSCLC.
PFL-721, a selective inhibitor targeting EGFR exon 20 and HER2 exon 20 mutations, is entering the dose-optimization phase of an ongoing first-in-human study in NSCLC. In parallel, PFL-241, a brain-penetrant fourth-generation EGFR inhibitor designed to overcome C797S resistance, is currently in the dose-escalation stage of its first-in-human trial.
Firmonertinib, a highly brain-penetrant, mutation-selective EGFR inhibitor, exhibits broad activity against common and rare EGFR mutations, including PACC and exon 20 insertions. It is under evaluation in a global Phase III trial for first-line treatment of EGFR exon 20 insertion-positive NSCLC (FURVENT; NCT05607550) and a Phase Ib trial including a cohort for EGFR PACC mutations (FURTHER; NCT05364073). Additionally, firmonertinib is being studied in combination therapy for advanced or metastatic NSCLC with classical EGFR mutations, through a collaboration between Allist Pharmaceuticals and ArriVent BioPharma.
PLB1004, a novel mono-anilino-pyrimidine small molecule, irreversibly inhibits EGFR exon 20 insertions while also demonstrating potent activity against common EGFR mutations such as ExDel19, L858R, and T790M, with high selectivity over wild-type EGFR.
The expected introduction of these therapies is set to drive growth in the EGFR NSCLC treatment market in the coming years while intensifying competition for Dizal’s recently approved ZEGFROVY.

Downloads
Article in PDF
Recent Articles
- ESMO Asia 2022: Role of EGFR and ALK mutations in the East Asian Lung Cancer Market
- Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZenec...
- Astellas & AviadoBio’s Exclusive Deal for AVB-101; GSK’s Depemokimab Shows Positive Res...
- 8 Emerging Bispecific Antibodies Transforming NSCLC Treatment
- Evolving Landscape for Rare Biomarkers in Non-Small Cell Lung Cancer



