AstraZeneca Strengthens Presence in PNH Treatment with Voydeya in Japan: World’s First Approval
Jan 26, 2024
A month following Novartis’ foray into the paroxysmal nocturnal hemoglobinuria (PNH) treatment domain, AstraZeneca strengthens its position in this field as its latest contender, Voydeya, secures a groundbreaking approval in Japan. Voydeya (danicopan), an innovative oral factor D inhibitor, has received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for its application in combination with a C5 inhibitor to address PNH patients who have not responded adequately to C5 inhibitors.
Paroxysmal nocturnal hemoglobinuria is a rare and serious blood disorder characterized by the breakdown of red blood cells within blood vessels, a condition known as intravascular hemolysis (IVH). Additionally, there is activation of white blood cells and platelets, leading to the potential formation of blood clots and subsequent organ damage, which may ultimately lead to premature death. The prompt, comprehensive, and sustained inhibition of the terminal complement pathway, achieved by blocking the C5 protein, proves effective in alleviating symptoms and minimizing complications, ultimately enhancing the survival rate for individuals with PNH. It’s noteworthy that about 10-20% of PNH patients undergoing treatment with a C5 inhibitor may still experience clinically significant extravascular hemolysis (EVH), resulting in ongoing anemia symptoms and necessitating blood transfusions.

DelveInsight’s analysis reveals that the overall diagnosed prevalent population of PNH in Japan was reported as ~950 in 2023. In Japan, the trend of prevalence in gender was observed differently as males were more affected by PNH rather than females which was analyzed in other remaining 7MM countries.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA grants approval; Sanaria receives fast-track designation; Sanofi and Regeneron’s next blockbu...
- Beyfortus: A New Respiratory Syncytial Virus (RSV) Drug for Toddlers
- The Business Cocktail : Latest Pharma deals
- Emergence of Stem cells and its Market Impact
- CHPM’s positive approval for 13 Medicines; NICE approval for AstraZeneca’s Olaparib; Exact Scienc...
Professor Jun-ichi Nishimura, MD, PhD, from the Department of Haematology and Oncology at Osaka University Graduate School of Medicine, expressed that the use of C5 inhibition has revolutionized the treatment of PNH. It has been demonstrated to effectively manage IVH, prevent complications, and enhance overall survival. Some patients undergoing C5 inhibitor therapy may encounter signs or symptoms of clinically significant EVH. In the ALPHA trial, the addition of Voydeya to Ultomiris or Soliris demonstrated improvements in hemoglobin levels, reduced the necessity for transfusions, and maintained control over IVH. The approval granted holds the potential to enhance outcomes for individuals grappling with the challenging manifestations of EVH while adhering to standard-of-care treatment.
The Phase III trial, known as ALPHA, investigated the effectiveness and safety of Voydeya as an additional treatment alongside Ultomiris or Soliris for patients with paroxysmal nocturnal hemoglobinuria (PNH) who had clinically significant extravascular hemolysis (EVH). This condition was defined as having a hemoglobin level of ≤9.5 g/dL and absolute reticulocyte count levels ≥120×109/L. The results demonstrated that Voydeya successfully achieved the primary objective, which was a change in hemoglobin levels from the baseline to week 12, and also met all key secondary objectives, including preventing transfusions and altering the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue) score. The findings from the ALPHA Phase III trial indicated that Voydeya was generally well-tolerated, with no new safety concerns identified. Throughout the trial, the most frequently reported treatment-related adverse events included headache, nausea, arthralgia, and diarrhea.
Marc Dunoyer, CEO of Alexion, expressed that over two decades of research on PNH has firmly established the effectiveness of C5 inhibition in treating this rare disease. The introduction of Voydeya as an additional treatment alongside standard care reflects our commitment to innovation for the PNH community. This development underscores our dedication to meeting the needs of individuals affected by clinically significant EVH, ensuring continuity with established therapies. We eagerly anticipate introducing this significant advancement to the specific subset of PNH patients in Japan grappling with this condition.
For an extended period, Alexion has been the primary player in the PNH treatment market through its products Ultomiris and Solaris. Recently, new contenders like Apellis’ Empaveli have emerged, and Novartis’ Fabhalta, a factor B inhibitor, obtained FDA approval in December. Notably, Fabhalta is the first oral monotherapy sanctioned in the US for the rare blood disorder. It’s worth mentioning that both Solaris and Ultomiris are administered through injections.
Meanwhile, Voydeya was designed to serve as a potential supplement to AstraZeneca’s existing C5 inhibitor treatments, Ultomoris or Soloris, which currently set the benchmark for standard care. Voydeya has received Breakthrough Therapy designation from the US Food and Drug Administration and has been granted PRIority MEdicines (PRIME) status by the European Medicines Agency. Additionally, Voydeya has obtained Orphan Drug Designation in the United States, European Union, and Japan for addressing PNH. Presently, Voydeya’s regulatory submissions are undergoing evaluation by various global health authorities.
Furthermore, Voydeya may face fierce competition from other pharma companies working in the PNH treatment domain. Hoffman la Roche in partnership with Chugai Pharmaceuticals is developing RG6107 or SKY59 (crovalimab), a humanized complement inhibitor C5 monoclonal antibody (anti-C5 recycling antibody) discovered by Chugai using recycling antibody technology. SKY59 is designed to target C5, a key component of the complement system, and is expected to control complement activity. It inhibits complement activation by blocking the cleavage of c5 to c5a and c5b, which is the cause of PNH. In September 2023, the FDA accepted the Biologics License Application (BLA) for crovalimab, an investigational anti-C5 recycling monoclonal antibody, based on positive results from the Phase III COMMODORE 2 study in PNH. Though the drug will be approved in the US, it will cause major damage to the sales of Voydeya.
BioCryst Pharmaceuticals, another company is also working with their lead asset BCX9930 to improve the PNH treatment market landscape. BCX9930 is a novel, oral, potent, and selective small molecule inhibitor of Factor D, discovered by the company and is currently in clinical development to treat complement-mediated diseases. Currently, it is in Phase II development for PNH treatment. In August 2020, the FDA granted ODD for BCX9930 treating PNH.
Omeros is also developing therapy for PNH treatment. Omeros lead candidate OMS906 is a prospective human monoclonal antibody designed to target mannan-binding lectin-associated serine protease-3 (MASP-3), a crucial activator of the alternative pathway within the complement system. The complement system plays a pivotal role in triggering inflammation, typically activated in response to tissue damage or microbial infections. MASP-3 is instrumental in converting pro-complement factor D to complement factor D and is considered a primary target in the alternative pathway. Notably, MASP-3 exhibits the lowest native circulating level and relatively low clearance compared to other alternative pathway proteins.
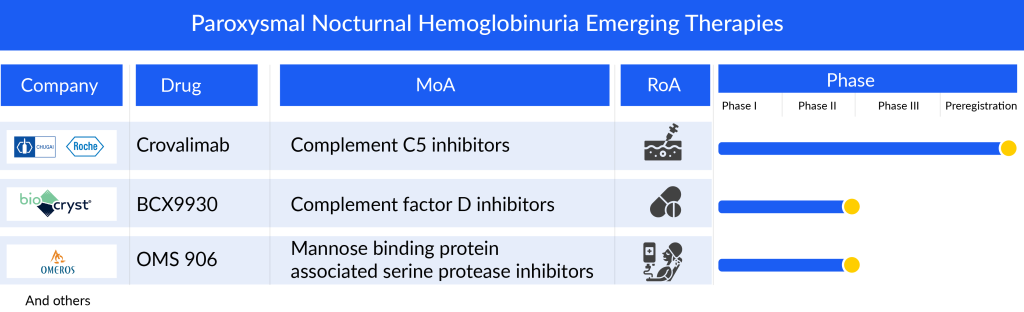
In contrast to inhibitors of C5 and C3, inhibiting MASP-3 does not compromise the lytic arm of the classical pathway, which is essential for combating infections. Another distinctive feature of MASP-3 is its presumed lack of association with acute phase reactions, providing a noteworthy advantage for MASP-3 inhibitors like OMS906 over alternative pathway inhibitors targeting other components.
In June 2023, the company revealed that information derived from a predetermined interim assessment of the ongoing Phase Ib clinical study involving OMS906, the principal MASP-3 inhibitor of the company, in adults with PNH who have not been treated with complement inhibitors, will be presented at the 2023 European Hematology Association (EHA) Congress in Frankfurt, Germany. Recognized as one of the top five last-minute submissions by the Scientific Program Committee, the summary was chosen for verbal delivery.
The anticipated launch of these therapies will not only boost the PNH treatment space forward but also be a reason for concern to Voydeya sales in the coming years. It will be very interesting to see how Voydeya will cope with this and stabilize its position in the PNH treatment market.
Downloads
Article in PDF
Recent Articles
- Driving factors boosting the Hepatocellular Carcinoma Market
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- AZ’s benralizumab; Sanofi, Regeneron set; Nordisk preps; European countries offer
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- Booming Healthcare sector in MENA: Lucrative opportunity for Global pharma players



