Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry
ResMed Acquired Somnoware, a Leader in Digital Sleep and Respiratory Care Diagnostics Software On July 05, 2023, ResMed acquired privately held Somnoware, a US leader in sleep and respiratory care ...

Astellas Announces FDA Grants Priority Review for Zolbetuximab Biologics License Application Astellas Pharma Inc. announced that the FDA has accepted and granted Priority Review for the company's Biologics Licence Application (BLA) for zolbetuximab, a first-in-class investigational Claudin 18.2 (CLDN18.2)-target...
Find More
Enovis completed the acquisition of Novastep On June 29, 2023, Enovis, one of the largest orthopedic device companies in the world, announced that the company had completed the purchase of Novastep and its foot and ankle minimally invasive surgical (MIS) platform, which was first announced in April 2023. ...
Find More
FDA Approves First Gene Therapy for Severe Hemophilia A BioMarin Pharmaceutical Inc., a global biotechnology company dedicated to transforming lives through genetic discovery, announced that the US Food and Drug Administration (FDA) has approved ROCTAVIAN (valoctocogene roxaparvovec-rvox) gene therapy for the tr...
Find More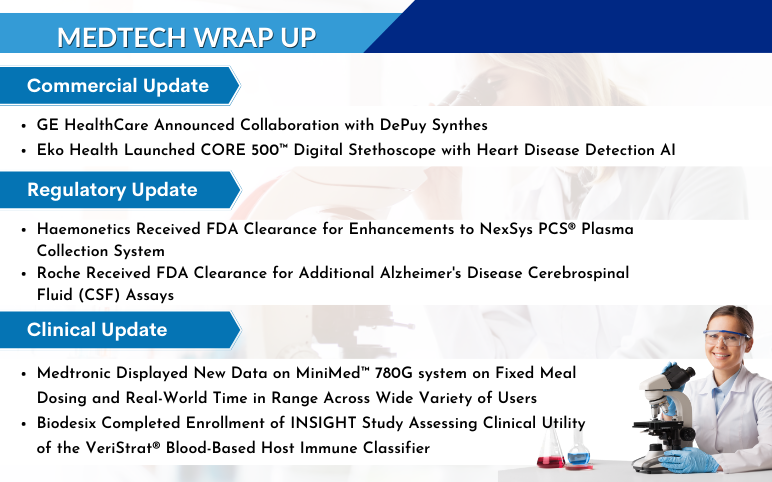
GE HealthCare Announced Collaboration with DePuy Synthes to Bring Advanced 3D Precision Imaging Innovation to Spine Practices in the United States On June 21, 2023, GE HealthCare entered into a distribution agreement with DePuy Synthes, the Orthopaedics division of Johnson & Johnson, to bring GE HealthCare’s...
Find More
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Find More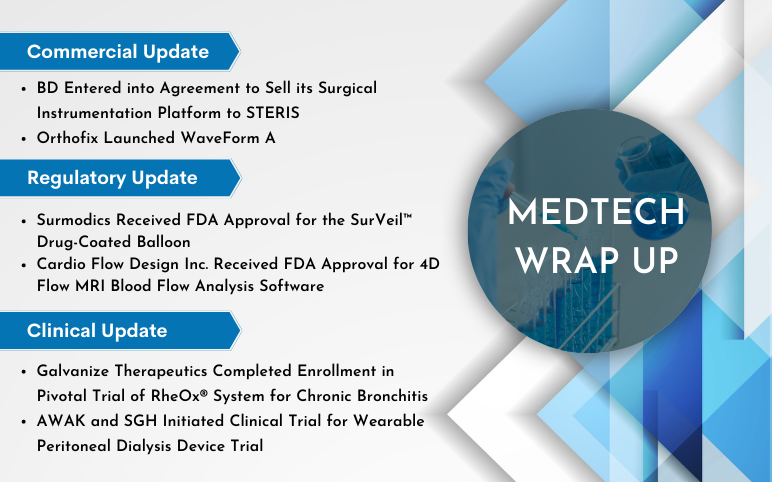
Orthofix Launched WaveForm A – 3D Printed Anterior Lumbar Interbody On June 19, 2023, Orthofix Medical Inc., a leading global spine and orthopedics company, launched WaveForm® A, an interbody for Anterior Lumbar Interbody Fusion (ALIF) procedures. For the treatment of patients who require fusion due to degenerat...
Find More
GSK Announces Extension of FDA Review Period for Momelotinib After all, GSK will not hear from the FDA this month about its marketing application for momelotinib as a therapy for anemia in myelofibrosis patients. The pharmaceutical company announced that the US Food and Drug Administration has extended the drug'...
Find More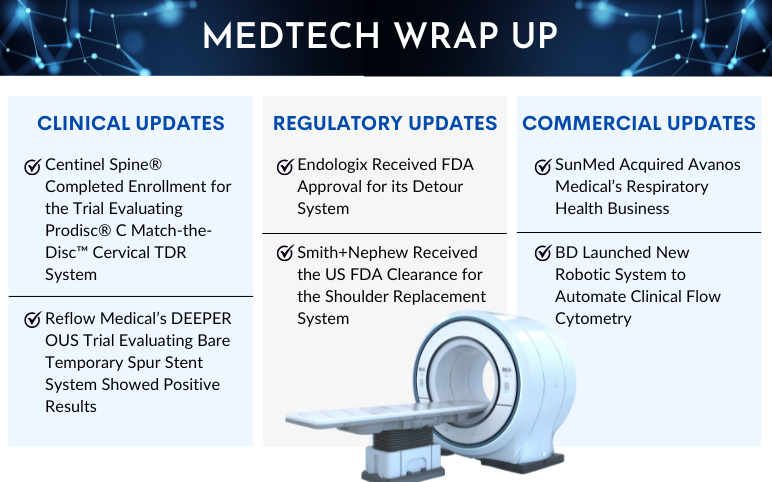
Centinel Spine® Completed Enrollment in First-of-its-Kind 2-Level IDE Trial Evaluating prodisc® C Match-the-Disc™ Cervical TDR System On June 13, 2023, Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-pr...
Find More
FDA Grants Fast Track Status to KYV-101 for Refractory Lupus Nephritis Treatment Kyverna Therapeutics announced that the FDA has given Fast Track status to KYV-101, a treatment for patients suffering from resistant lupus nephritis (LN). KYV-101 is an innovative therapy that uses anti-CD19 chimeric antigen recept...
Find More
Lean is a multi-faceted framework that allows organisations to exercise different initiatives simult.....
Find More
Intra-Tumoral Cancer Therapy refers to any therapy that is delivered in very close anatomical proxim.....
Find More
There are about 200 different types of cancer, making it one of the most diverse types of indication.....
Find More
Digital health technologies can fill the current gaps in oncology care while rebalancing the existin.....
Find More
Fragile X Syndrome is a syndrome, which results in intellectual disabilities as well as affects the .....
Find More
Major Depressive Disorder (MDD), also known as clinical depression, is a severe, debilitating and fr.....
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.