Oncolytic Viruses: Can Be The Next Frontier in Cancer Immunotherapy?
Oct 17, 2022
Table of Contents
Oncolytic viruses are cancer treatments that employ a natural or reprogrammed virus capable of targeting and killing malignant cells. They are a new class of cancer agents that induce tumor regression by causing immunogenic cell death, inducing preferential replication in tumor cells, and stimulating host antitumor immunity. Oncolytic viruses are experimental cancer treatments that try to employ viruses’ intrinsic features to help fight cancer. These non-human viruses proliferate primarily in cancer cells while disregarding healthy ones.
Oncolytic viruses can create virions fast and genetically design new genes that boost antitumor immunity, make tumor cells more susceptible to ionizing radiation or cytotoxic treatment, and improve patient safety.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- ESMO Asia 2022: Role of EGFR and ALK mutations in the East Asian Lung Cancer Market
- CAR-T in killing tumors; Amunix raises $73M; Autism findings in protein-targeted treatment
- Medtronic Gains CE Mark for LigaSure™ on Hugo™ Robotic System; J&J Wins FDA Nod for VARIPULSE...
- Liso-cel (lisocabtagene maraleucel) Shines in Second-Line, Yielding Long-Lasting Complete Respons...
- Niacin fights Glioblastoma, iTeos nets $125M, Pandion reels in $80M, Brii, Chinese partner, FDA t...
Vectors Used in Oncolytic Virotherapy
Several DNA and RNA viruses may be utilized as oncolytic viruses, with DNA viruses being employed in the bulk of clinical research. Adenovirus, HSV-1, reovirus, and poxviruses were among the most frequent viruses.
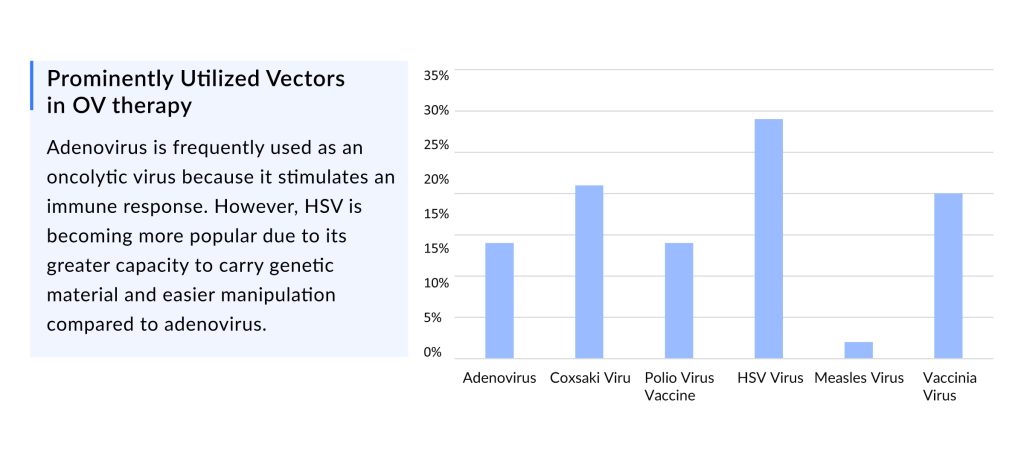
Adenovirus is one of the most commonly utilized oncolytic viruses due to its immune response stimulation. Still, HSV is now being used more because it has a more extensive viral ability to carry genetic information and is more easily manipulated than adenovirus.
HSV-1 is harmful to humans and can induce mucosal or skin infections and central nervous system infections, indicating the necessity for further transgene deletions and insertions to generate viable oncolytic virus therapy.
Routes for Delivering Oncolytic Virus Therapies
The route of administration of oncolytic viruses are inherently tied to the kind of tumor to be treated, given that the viral pathway directly affects the success of the therapy owing to virus availability on-site and the natural barrier of the organism to fight against antigens. The distribution can be intraperitoneal, intrathecal, subcutaneous, intratumoral, and intravenous, which is connected to the treatment of distant metastases. Viruses are chosen or changed to target tumor cells, reduce pathogenicity to normal cells, reduce the antiviral immune response (to prevent viral clearance), and raise the antitumor immune response.
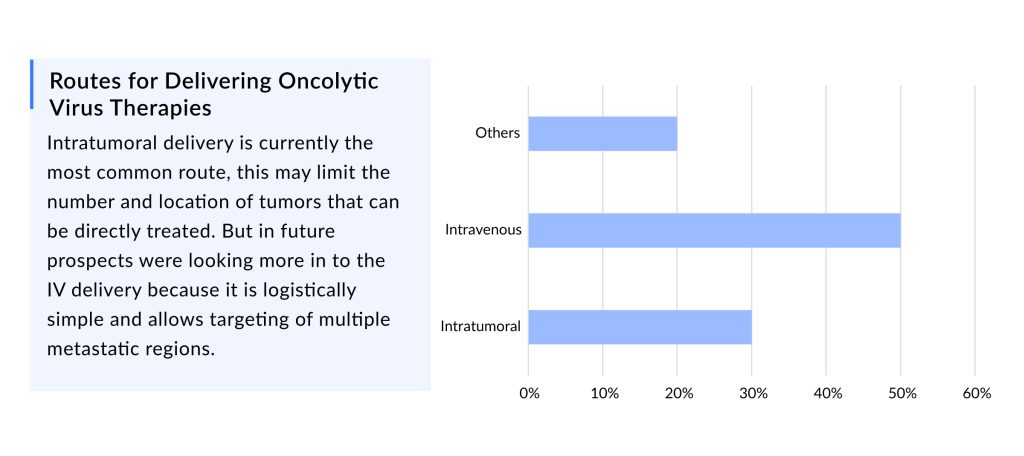
Intratumoral administration is currently the most prevalent approach, which may limit the number and location of tumors that may be directly treated. However, prospects were interested in IV delivery in the future since it is logistically straightforward and enables the targeting of various metastatic regions.
Approved Oncolytic Viruses in Cancer Therapy
Imlygic -talimogene laherparepvec (T-VEC)
Based on a Phase III OPTiM study that demonstrated its effectiveness against GM-CSF alone, Imlygic was approved in 2015 for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after the first surgery.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Around the same time, PD-1 checkpoint inhibitors were approved, which significantly increased survival periods for patients with melanoma, making it difficult for Amgen to explain its trial design with GM-CSF as the comparison arm.
Another setback occurred when the findings of the OPTiM study were not very outstanding, as the medicine only delayed mOS by 4.4 months, resulting in the loss of the US FDA Fast Track designation. However, patients who had a lasting response in the first year of therapy had a 95% lower chance of mortality, which ultimately helped Amgen get approval.
DelveInsight’s Opinion: Despite being the first in class, the uptake of T-VEC has remained slow due to the limited patient pool for which it is suitable (~10% of patients who are ineligible for surgery are target candidates for Imlygic) and the limited efficacy of the drug compared to the other immunotherapies currently available for melanoma patients. Considering these limitations, we project Imlygic to, at best, generate revenue of a maximum of USD 150 million by 2032 in the 7MM.
DELYTACT -teserpaturev
Delytact (teserpaturev) is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1) that has gained conditional and time-limited marketing authorization in Japan for malignant glioma treatment. It is the world’s first oncolytic virotherapy for brain cancer treatment.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Data from a single-arm phase II study demonstrated that Delytact enhanced the odds of survival at one year in patients with residual or recurrent glioblastoma who had previously been treated with radiation and temozolomide chemotherapy.
DelveInsight Opinion: DELYTACT has received conditional and time-limited marketing approval for 7 years in Japan only. Currently, it is commercially available only at hospitals that serve as trial sites, and Daiichi Sankyo will establish a stable supply system of medicine as soon as possible. Considering these limitations, we estimate that DELYTACT, at best, will generate a revenue of a maximum of USD 51 million in 2032 in the 7MM.
Potential Oncolytic Viruses in Cancer Therapy Under Clinical Development
Approximately 12 tumor types are currently being targeted, with a potential patient pool of 1 billion. More than 15 leading biotech and pharmaceutical companies in the seven major markets, including Targovax, Replimune, Genelux Corporation, Candel Therapeutics, DNAtrix, SillaJen, Treovir, Lokon Pharma AB, Istari Oncology, CG Oncology, Amgen, Daiichi Sankyo, and others are currently evaluating novel oncolytic virus cancer therapies to cater to the need of the patients.
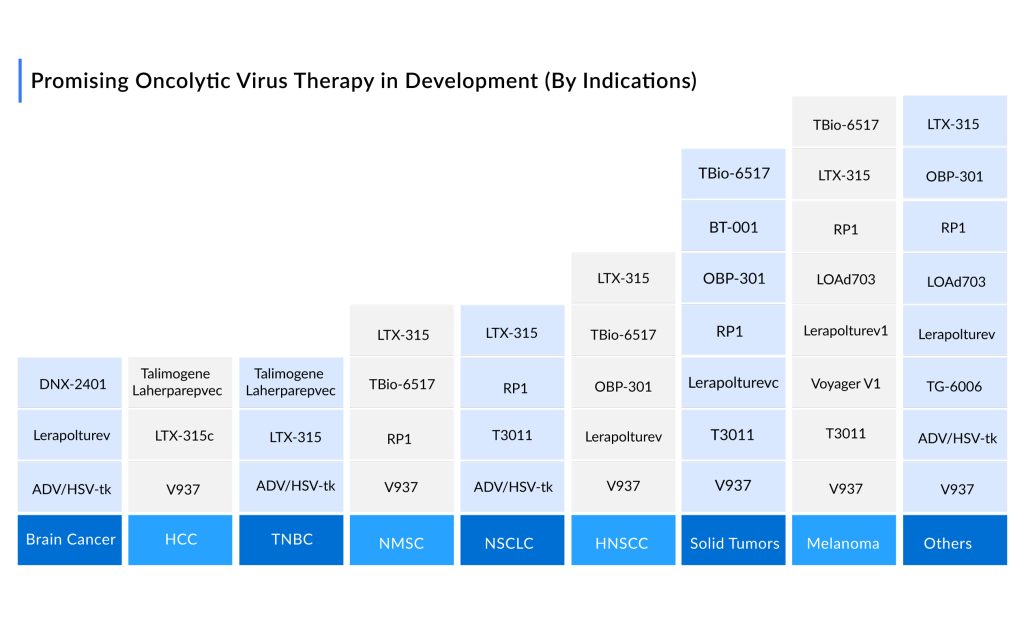
The pipeline for oncolytic viruses for cancer therapy is robust, with more than ~ 30 assets in Phase II and above the stage of development. The promising oncolytic cancer therapies in the pipeline include ONCOS-102, RP1 (Vusolimogene Oderparepvec), GL-ONC1, CAN-2409, DNX-2401 (Tasadenoturev), PEXA-VEC (Pexastimogene Devacirepvec; JX-594), G207, LOAd703, Lerapolturev (Formerly Known as PVSRIPO), CG0070, and others.
Will DNX 2401 (tasadenoturev) + Pembrolizumab Combo be a Gamechanger For Cancer Treatment?
DNX-2401 (DNAtrix) is an oncolytic immunotherapy developed to meet the twin requirements of high potency and safety. To accomplish this, two stable genetic modifications in the adenovirus genome were made such that it replicates exclusively in Rb pathway defective cells and efficiently infects tumor cells.
Pembrolizumab developed by Merck is a humanized monoclonal anti-programmed cell death-1 (PD-1) antibody (IgG4/kappa isotype with a stabilizing sequence change in the Fc region) generated by recombinant DNA technology in Chinese hamster ovary cells.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DNAtrix, in collaboration with Merck Sharp & Dohme Corp., currently planned a Phase III trial for DNX-2401 plus pembrolizumab combo for recurrent glioblastoma treatment with the trial acronym “IGNITE.”
Analyst Comments: Overall, these findings provide the first clinical evidence that DNX-2401 can provide a direct oncolytic impact followed by an antitumor immune response. Insofar as DNX-2401 is extremely effective due to increased infectivity afforded by the RGD motif, it is capable of increasing antigen presentation; we infer that these actions contribute to immune cell infiltration and anticancer activity.
Recent Developments in Oncolytic Viruses for Cancer Therapy
- In June 2022, Targovax announced that the US FDA approved an IND application for TG01 combined with QS-21 STIMULON, which allows the preparations to initiate clinical trials in the USA to proceed.
- In May 2022, KaliVir Immunotherapeutics, Inc. announced a collaboration and global exclusive licensing agreement with Roche for the discovery, development, and commercialization of novel oncolytic vaccinia viruses derived from KaliVir’s oncolytic immunotherapy VETTM platform.
- In May 2022, Calidi Biotherapeutics had granted a new patent (No. US 11,285,194, Combination immunotherapy approach for cancer treatment) by the US Patent and Trademark Office (USPTO) related to its proprietary SuperNova (SNV) technology platform. The enhanced oncolytic virotherapy delivery platform was designed to provide and improve therapeutic treatments for multiple cancer indications. Calidi’s product candidates are in the early stages of development and have not yet been approved by the US FDA.
- In March 2022, Synthetic Biologics, Inc. announced that it had completed the acquisition of VCN Biosciences, S.L. (VCN), following the satisfaction of all closing conditions.
- In January 2022, Transgene and PersonGen BioTherapeutics announced a strategic collaboration to evaluate the feasibility and efficacy of combination therapy associating PersonGen’s TAA06 CAR-T cell injection with intravenous (IV) administration of an armed oncolytic virus from Transgene’s Invir.IO™ platform, in solid tumors including pancreatic cancer and brain glioma. The collaboration aimed to demonstrate the combination’s likely synergistic mechanisms to potentiate CAR-T cell therapy. Under the terms of the collaboration agreement, Transgene to develop multiple new oncolytic virus candidates, using its patented oncolytic virus backbone VVcopTK-RR- and its Invir.IO™ technology platform, specifically for IV administration in combination with PersonGen’s TAA06 CAR-T injection. PersonGen to evaluate the efficacy of the combination to eliminate solid tumors in preclinical models.
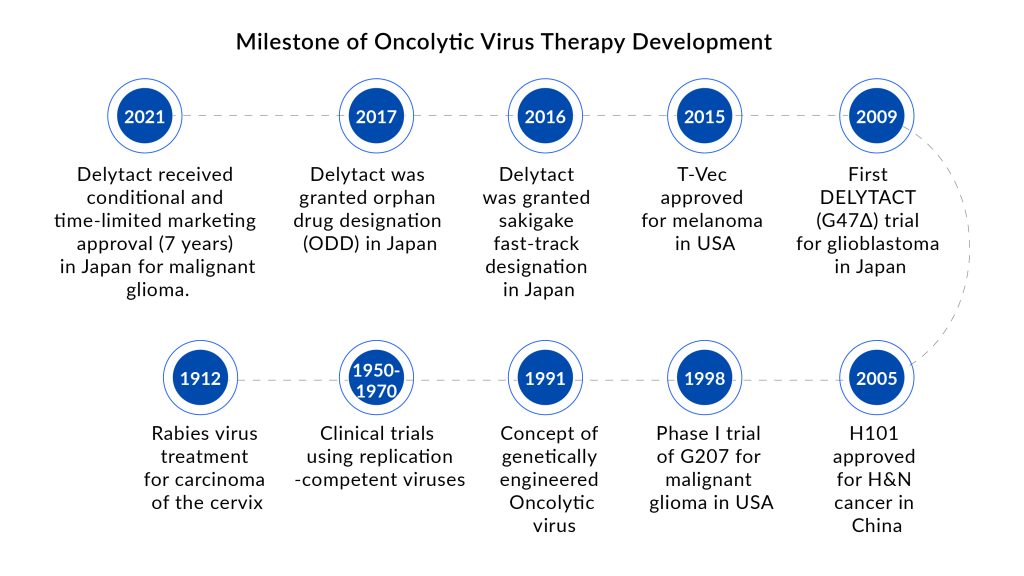
Future Perspectives of Oncolytic Viruses in Cancer Therapy
The approval of multiple drugs targeting various cancer indications is predicted to change the dynamics of the oncolytic cancer therapies market in the future years.
According to DelveInsight analysis, the oncolytic cancer therapies market size in the 7MM was approximately USD 122 million in 2021, which is further expected to increase at a CAGR of 32.9% by 2032. As per our estimates, the United States will have the largest oncolytic virus therapies market share.
Furthermore, oncolytic viruses offer several benefits over conventional tumor immunotherapies, such as high killing effectiveness, precision targeting, fewer side effects or drug resistance, and low cost, which is propelling the oncolytic virus therapies market growth. Moreover, as some oncolytic viruses, such as Adeno oncolytic viruses, exhibit antitumor memory, they might be employed as a cancer vaccine.
In addition, there is an opportunity for blockbuster drugs like Keytruda/Opdivo and other IOs to target a huge patient pool of checkpoint-resistant patients (50-60% of their present patient pool becomes refractory).
Moreover, considerable novel techniques, such as the utilization of nanoparticles, complex viral particle ligands, liposomes, polymeric particles, and immunomodulatory drugs, have been developed to investigate effective oncolytic virus delivery methods.
Furthermore, existing knowledge and experience with immunotherapy create a compelling argument for institutional approval of oncolytic virus cancer therapies. Immunotherapy is being used in roughly 30 different cancer types, which will provide a large patient pool for novel emerging therapies in the coming years.

FAQs
Oncolytic viruses (OVs) are a type of anti-cancer agent that promotes tumor regression by preferentially replicating in tumor cells, inducing immunogenic cell death, and stimulating antitumor immunity in the host. It employs both native and reprogrammed viruses to target and destroys cancerous cells.
Oncolytic viruses are a new class of cancer agents that induce tumor regression by causing immunogenic cell death, inducing preferential replication in tumor cells, and stimulating host antitumor immunity. Oncolytic viruses are experimental cancer treatments that try to employ viruses’ intrinsic features to help fight cancer. These non-human viruses proliferate primarily in cancer cells while disregarding healthy ones.
There are only two oncolytic virus cancer therapies available in the market, namely, IMLYGIC (Talimogene laherparepvec) (Amgen) and Delytact (Auidyw) (Daiichi Sankyo).
More than 15 leading biotech and pharmaceutical companies, including Targovax, Replimune, Genelux Corporation, Candel Therapeutics, DNAtrix, SillaJen, Treovir, Lokon Pharma AB, Istari Oncology, CG Oncology, Amgen, Daiichi Sankyo, and others are currently evaluating novel oncolytic virus cancer therapies to cater to the need of the patients.
The promising oncolytic cancer therapies in the pipeline include ONCOS-102, RP1 (Vusolimogene Oderparepvec), GL-ONC1, CAN-2409, DNX-2401 (Tasadenoturev), PEXA-VEC (Pexastimogene Devacirepvec; JX-594), G207, LOAd703, Lerapolturev (Formerly Known as PVSRIPO), CG0070, and others.
Downloads
Article in PDF
Recent Articles
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
- Liso-cel Shines in TRANSCEND FL: Impressive Complete Responses, Durable Overall Responses, and Ma...
- New cancer drug tested in mice may benefit certain leukaemia patients
- Incyte’s Clinical Trial for Myelofibrosis; Eisai and Bioge’s Leqembi; FDA Approves Reata Pharmace...
- The Rise of TCR Therapy: A Beacon of Hope in Cancer Treatment