How to Cure Treatment-resistant Depression?
Feb 14, 2022
Table of Contents
Treatment-resistant Depression (TRD) typically refers to inadequate response to at least two antidepressant medications used for a sufficient length of time at an adequate dose with an adequate affirmation of treatment adherence. Treatment-resistant Depression is a relatively common occurrence in clinical practice, with 50–60% of the patients not achieving sufficient response following antidepressants for Treatment-resistant Depression.
Treatment-resistant Depression is usually linked to higher rates of comorbidity, particularly with other psychiatric disorders, chronic pain, and fibromyalgia. On the other hand, the impact of both physical and psychiatric illness comorbidities is much higher amongst the patients. Some environmental factors related to Treatment-resistant Depression are lower socioeconomic status, non-supportive social environment, family conflicts, chronic stressors, multiple loss events, lower level of education, and social support and work dysfunction.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- How is Artificial Intelligence (AI) Playing a Constructive Role in Mental Health Management?
- Narcolepsy now a confirmed Autoimmune Disorder
- How Depression Is Eroding The Mental And The Physical Health Of The Society?
- Major Depressive Disorder Market Quadrant Growth Soars Owing to Expected Launch of Key Therapies
- Plotting the Extensive Demand of Mobile Apps for Mental Health
Treatment-resistant Depression Epidemiology
To determine the prevalent population of Treatment-resistant Depression in the 7MM (United States, Germany, France, Spain, Italy, United Kingdom, and Japan), we considered and compared data from various studies and organizational databases. Along with this, we also collected different KOL views and expert opinions to cover the geographical differences in Treatment-resistant Depression epidemiology across the 7MM.
The total 7MM prevalent cases of Treatment-resistant Depression stood at nearly 5.8 million in 2021. The United States accounted for the highest number of cases, followed by EU5 countries and Japan. Japan had ~270,000 cases of Treatment-resistant Depression in 2021, while the EU5 countries together accounted for over 1.5 million cases, as per DelveInsight’s assessments.
According to DelveInsight’s estimates, among the European countries, Germany had the highest number of prevalent cases of Treatment-resistant Depression (~560k) in 2021 and is expected to reach ~550k by 2032 owing to a declining population. At the same time, Italy had the lowest number of prevalent cases (~127k) in 2021, which as per DelveInsight’s estimates, are expected to reach nearly 130k cases by 2032.

Treatment-resistant Depression Market
According to DelveInsight, the lion’s share of the Treatment-resistant Depression market remains with the United States throughout the forecast period (2019–2032). The Treatment-resistant Depression market size in the US was ~USD 1,800 million in 2019 and is likely to reach ~USD 9,000 million by 2032.
In 2019, among EU5, Germany accounted for the largest market size (USD 150+ million), followed by the UK with ~USD 105 million,whereas Italy accounted for the smallest (~USD 35 million).
The current therapeutic options Aripiprazole (Abilify), Brexpiprazole (Rexulti) or Quetiapine (Seroquel XR) are FDA-approved as add-on therapies to an antidepressant for Treatment-resistant Depression. The future of the Treatment-resistant Depression market looks promising with emerging therapies such as AXS-05 (Axsome Therapeutics), Cariprazine (AbbVie), Esketamine DPI (Celon Pharma), AV-101 (VistaGen Therapeutics), REL-1017 (Relmada Therapeutics, Inc. and Syneos Health ), and MIJ821 (Novartis Pharmaceuticals).
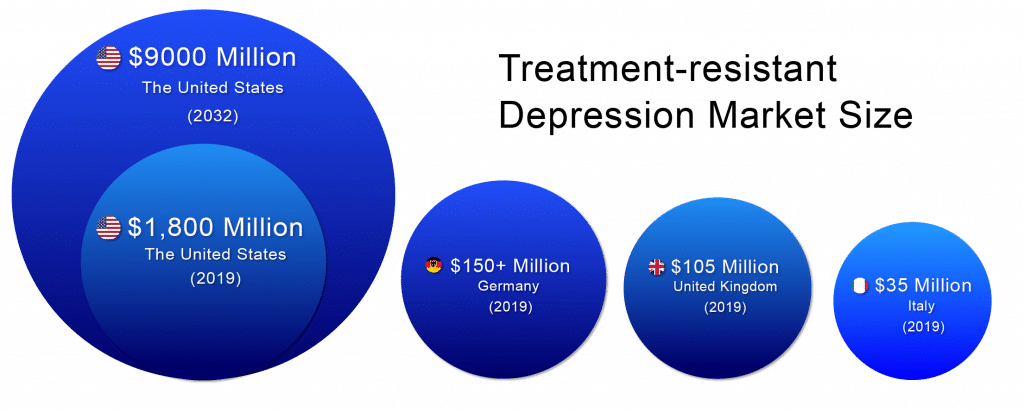
Market Leaders in the Making
With a cornucopia of drugs in the Treatment-resistant Depression pipeline, market size is projected to increase. Among the emerging therapies, the most promising ones include AXS-05 (Axsome Therapeutics) – a Phase II NMDA receptor antagonist with multimodal activity, Esketamine therapy – a Phase II NMDA antagonist, and REL-1017 – Phase III NMDAR Antagonist, among others.
Treatment-resistant Depression Therapeutic Trends
Pharmacologic treatment options include switching, combination, and potentiation strategies among commonly used antidepressant drugs. Most of the available antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), dual serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, irreversible, non-selective monoamine oxidase inhibitors (MAOIs), α2-antagonists, agomelatine, and tianeptine are used to treat TRD.
Non-pharmacological therapies include electroconvulsive therapy (ECT), Repetitive transcranial magnetic stimulation (rTMS), and Vagus nerve stimulation (VNS). Along with this, two alternative forms of psychotherapy involve majorly cognitive-behavioural therapy (CBT) and interpersonal therapy (IPT).
A relatively wide variety of treatment options for unipolar Treatment-resistant Depression are available, whilst the evidence is very scanty for bipolar TRD. Treatment-resistant Depression is associated with poor clinical, functional, and social outcomes. Several novel drug therapies that could tackle Treatment-Resistant Depression are currently being investigated as promising alternatives, targeting the neurotransmitter system outside the standard monoamine hypothesis. Augmentation or combination with lithium or atypical antipsychotics appears as a valid option for both conditions, and the same occurs with electroconvulsive therapy. Other non-pharmacological strategies, such as deep brain stimulation for Treatment-resistant Depression may be promising alternatives for the future. Cognitive behaviour therapy is recommended for unipolar TRD, but there is no evidence supporting its use in bipolar Treatment-resistant Depression.
Disease associations and other organized programs for Treatment-resistant Depression patients are established in most regions. Some prominent ones include the Vandrevala Foundation, Anxiety and Depression Association of America, Substance Abuse and Mental Health Services (SAMHSA), National Alliance on Mental Illness (NAMI), and Anxiety and Depression Association of America (ADAA). These Treatment-resistant Depression support groups and organizations provide opportunities to map the patients and improve their access to innovative treatments and outcomes. Soon, establishing country-wide registries in cooperation with these centres may also help tackle the disease further.
Before deciding that a patient is treatment-resistant, physicians should assess the adequacy of treatment by determining adherence to the Treatment-resistant Depression medication. Non-adherence is estimated to be between 20% and 50% of all patients. It is prevalent in persons with cognitive defects and feelings of worthlessness and hopelessness. Ongoing cognitive behaviour therapy (CBT) and frequent follow-up with the physician may encourage adherence during the early stages of treatment. In patients who have been determined adherent, obtaining serum drug levels may be helpful, especially in those taking tricyclic antidepressants. Particular attention should be paid to comorbid substance abuse or other psychiatric conditions because they can significantly complicate the treatment of the underlying depression.

Treatment-resistant Depression Roadblocks
Patients with Treatment-resistant Depression may be affected by varying factors such as genetic, epigenetic, endocrine, and environmental factors, such as traumatic or stressful life events, which could collectively contribute to the heterogeneity of Major Depressive Disorder (MDD) or Treatment-resistant Depression. The exact biological pathophysiology behind MDD is unknown; the major focus of treatment until now has been targeting monoamine pathways. The monoamine theory recommends that depression results from a deficiency in one or more monoamines (serotonin, norepinephrine, and dopamine). However, monoamine pathways may be a factor of a much more intricate system of neural pathways that could form the basis of Major Depressive Disorder and resistance to treatment. Several pathways and biological procedures may have a role in the disease of MDD, including pathways that regulate serotonin, acetylcholine, dopamine, GABA, glutamate, opioid, norepinephrine, and vasoactive intestinal peptide, and have implications for patients with Treatment-resistant Depression.
The treatment response of patients suffering from Treatment-resistant Depression varies from patient to patient. According to various researches, 10–30% of patients who take antidepressants for Treatment-resistant Depression experience a poor response with residual symptoms and are required to try a variety of treatment alternatives. As the disease course of TRD is variable, different patients may experience major depressive episodes (MDEs) of varying severity, frequency, and characteristics of symptomatic, which may affect treatment response. The clinically significant benefit of treatment depends on the initial severity of TRD, the degree of resistance to the antidepressant medication, and the rating scale used to assess benefits in symptom enhancements. Even with multiple antidepressant medications, patients with Treatment-resistant Depression do not always attain remission and often experience residual symptom burdens. Continued residual symptoms are associated with a higher risk of relapse, highlighting the urgent unmet need to manage for better long-term outcomes.
Treatment-resistant Depression represents a chronic and complex illness that requires long-term management from medical specialists, which requires complete knowledge of adequate medication dose and duration. The retrospective studies from the health services database are frequently used to define the epidemiology and cost of Treatment-resistant Depression. The clinical information available in health services databases is quite limited and does not reflect the real-time clinical status of patients. Moreover, there is confusion in defining TRD, making it difficult to predict the exact prevalence and incidence rates across regions. Additionally, the discrepancy between diagnostic criteria and staging using different assessment scales is a source of confusion across multiple research settings. Hence, there is an urgent need to collect nationwide real-time data on this serious condition.
Treatment-resistant Depression is widely diagnosed as MDD that does not respond or remit to one or more antidepressant treatments of adequate dose and duration. Nierenberg and Amsterdam introduced the term “pseudo-resistance” for indicating non-responsiveness in MDD patients due to reasons including the presence of inappropriate dose and duration of treatment, poor adherence by the patient, or important comorbidity that may affect response.
Future of Treatment-resistant Depression
As per DelveInsight’s estimates, the Treatment-resistant Depression market landscape looks promising, studded with remarkable advances in diagnosis and treatments that will translate into a better quality of life and improved survival. The entry of the novel therapies to attain better healthcare will necessitate collaboration among academia, life-sciences companies, funding, regulatory agencies, decision-makers, and caregivers. In addition, expertise in navigating market access and reimbursement scenarios would be an elixir to devise successful GTM strategies for new therapies.

FAQs
Treatment-resistant Depression (TRD) refers to inadequate response to at least one antidepressant trial of adequate doses and duration.
Some medical conditions like heart disease, cancer, anorexia, or thyroid problems can contribute to Treatment-resistant Depression.
Olanzapine-Fluoxetine (Symbyax) is a combination drug that contains the active ingredients in fluoxetine (Prozac) and olanzapine (Zyprexa) together in one tablet and is approved for Treatment-resistant Depression.
Aripiprazole (Abilify), Brexpiprazole (Rexulti) or Quetiapine (Seroquel XR) are FDA-approved as add-on therapies to an antidepressant for Treatment-resistant Depression.
Downloads
Article in PDF
Recent Articles
- J&J’s SPRAVATO Achieves Milestone with Monotherapy Approval in Depression Treatment
- Will a Robust Pipeline Address the Major Depressive Disorder Treatment Conundrum?
- Psychedelic-inspired medicine – A wonder drug for generalized anxiety?
- Watch Out For Top Pipeline Therapies Making An Impact In The Bipolar Depression Market
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs