All Posts

BeiGene’s BRUKINSA Gets FDA Accelerated Approval; GSK’s Positive Results in DREAMM-8 Phase III; Sandoz’s Denosumab Biosimilars FDA Approved; Terns Pharma’s TERN-701 Receives Orphan Drug Designation; Wegovy® Approved in US for Overweight Cardiovascular Risk; Travere Therapeutics Submits sNDA for FILSPARI IgAN Full Approval
BeiGene Receives FDA Accelerated Approval for BRUKINSA in Relapsed/Refractory Follicular Lymphoma BeiGene, Ltd., has declared that the FDA has provided accelerated approval for BRUKINSA® (zanubrutinib) to be used in treating adult patients with relapsed or refractory (R/R) follicular lymphoma (FL), when used alo...
Find More
Giant-Cell Arteritis Treatment Beyond Glucocorticoids: Exploring Horizons
Giant-cell arteritis (GCA), alternatively referred to as temporal arteritis, is a form of vasculitis primarily impacting arteries, particularly those in proximity to the temples. If left untreated, it may result in notable complications such as loss of vision. Diagnosis of the disease is often complicated by its pr...
Find More
Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
Alport syndrome, an inherited disease, predominantly manifests in its X-linked form, constituting around 80% of cases. Without intervention, roughly 90% of affected males face kidney failure by age 40, whereas females typically experience a slower progression to this condition. Many Alport syndrome cases go unnotic...
Find More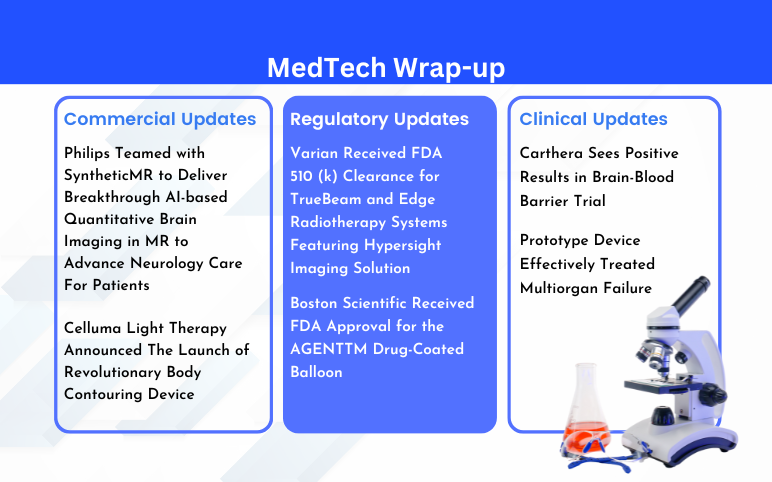
Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGENTTM Drug-Coated Balloon; Varian’s TrueBeam and Edge Radiotherapy Systems; Carthera’s Brain-Blood Barrier Trial; Prototype Device Effectively Treated Multiorgan Failure
Philips Teamed with SyntheticMR to Deliver Breakthrough AI-based Quantitative Brain Imaging in MR to Advance Neurology Care For Patients On March 01, 2024, Philips in collaboration with SyntheticMR announced the launch of Smart Quant Neuro 3D, a significant advancement in objective decision support for diag...
Find More
How Innovations Are Driving the Growth of the Nicotine Replacement Therapy Market?
The Nicotine Replacement Therapy (NRT) market is experiencing a notable evolution driven by a confluence of factors, including shifting societal attitudes towards smoking cessation, heightened awareness of the health risks associated with tobacco use, and an increasing emphasis on promoting public health initiative...
Find More
Major Highlights and Insights from the AAAAI Annual Meeting, 2024
American Academy of Allergy, Asthma & Immunology (AAAAI) 2024 annual meeting, is the premier educational event for allergists/immunologists around the world that was held from February 23 to 26 at the Walter E. Washington Convention Center this year. The American Academy of Allergy, Asthma & Immunology (AAA...
Find More
Bayer’s New Cardiology Drug Acoramidis; Two Datopotamab Deruxtecan Applications Validated in the EU; AbbVie and OSE Immunotherapeutics Announce Announces Partnership; vTv Therapeutics Makes Major Move With Cadisegliatin; A2 Bio Scores FDA Orphan Drug Designation for its Therapy, A2B530; FDA Fast Track Designation for AlloNK® in Lupus Nephritis
Acoramidis Joins Bayer's Robust Lineup, Boosting Cardiology Solutions Bayer has obtained the exclusive rights to market acoramidis in Europe from Eidos Therapeutics Inc., BridgeBio International GmbH, and BridgeBio Europe B.V. Acoramidis, a highly potent and selective small molecule given orally, functions as a ...
Find More
Gene Therapies as a Game-Changer in Ophthalmology: Eyeing the Future
Gene therapy is becoming a promising solution for retinal degenerative illnesses, particularly because the retina offers an excellent avenue for studying and treating eye-related issues. Importantly, it is the first tissue in the United States to receive approval for gene therapy in cases of inherited disorders. ...
Find More
Revolutionizing the Food Allergy Treatment: The Impact of Xolair’s Approval
Individuals suffering from food allergies now have access to a medication that can potentially avert serious consequences. This medication, which has been available for twenty years, has received approval from the FDA. Xolair (omalizumab) by Roche and Novartis is the first food allergy treatment sanctioned to dimin...
Find More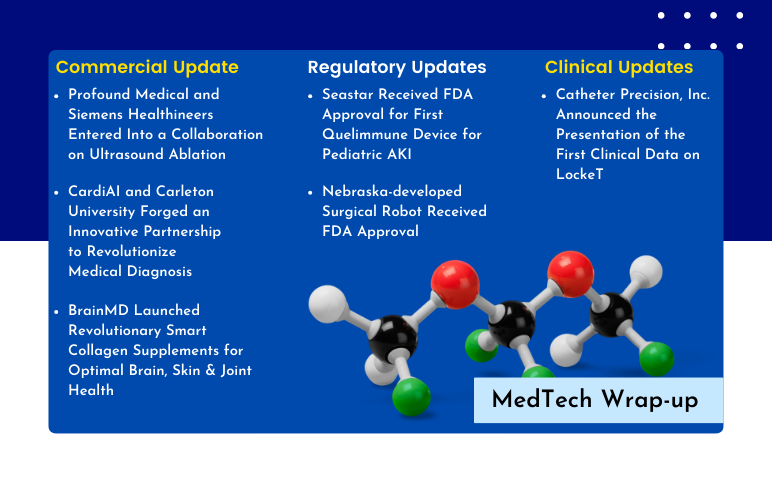
Profound Medical and Siemens Entered Into a Collaboration; CardiAI and Carleton University Forged an Innovative Partnership; BrainMD Launched Revolutionary Smart Collagen Supplements; FDA Approved Seastar’s Quelimmune Device; Nebraska-developed Surgical Robot Received FDA Approval; Catheter Precision Presentated First Clinical Data on LockeT
Profound Medical and Siemens Healthineers Entered Into a Collaboration on Ultrasound Ablation On February 27, 2024, Profound Medical entered into a non-exclusive agreement with Siemens Healthineers. The objective of the agreement was to establish the foundation for Profound's commencement of marketing a comprehe...
Find MoreEditor's Pick
Newsletter/Whitepaper
ASCO Conference 2023

The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.






-Agonist.png)

