Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

AgomAb Therapeutics raises USD 23.5 million for its agomAbs A Belgian biotherapeutics company AgomAb Therapeutics which has been in a long run of making agonistic antibodies has secured USD 23.5 million in a Series A financing round from international investor syndicate co-led by V-Bio Ventures (Belgium) and Adv...
Find More
Tetra, Shionogi sign USD 40 Million deal for Alzheimer’s disease and fragile X syndrome Tetra Discovery Partners is collaborating with Shionogi to progress its PDE4-targeting drug in Alzheimer’s disease and fragile X syndrome. Shionogi will give a USD 5 million upfront payment and USD 35 million in equ...
Find More
Lilly remunerates AC Immune USD 81 Million for preclinical Alzheimer’s drug Eli Lilly has remunerated USD 81 million upfront for the global rights to AC Immune’s tau aggregation inhibitors in Alzheimer’s disease. The agreement provides Lilly ownership of a small molecule that has prevented tau aggregation in preclin...
Find More
Novartis signs USD 2.1Billion for Endocyte acquisition Novartis has inked USD 2.1 billion agreement to takeover Endocyte. Endocyte, a biopharmaceutical company, headquartered in Indiana, licensed the cancer drug that catches Novartis’ eye for USD 12 million. Endocyte was in the stagnation when it held its recovery h...
Find More
Aimovig of Amgen commence robust for migraine treatment The U.S. Food and Drug Administration (FDA) has given the approval to preventative migraine treatment, Aimovig of Amgen. Aimovig has a price of USD 6,900 per year, but according to analysis of sales, it gives information that migraine treatment is making its wa...
Find More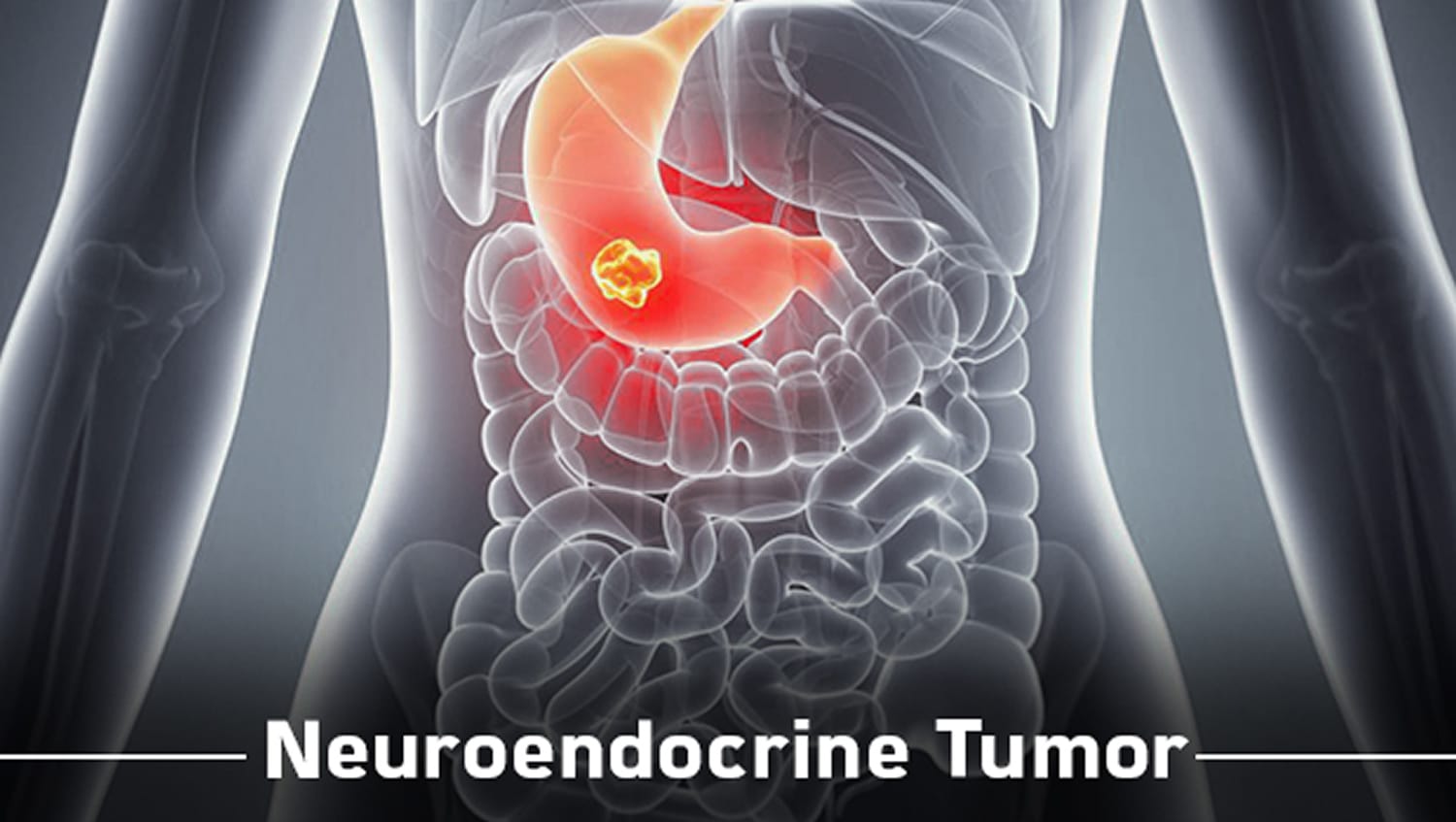
Neuroendocrine tumors (NETs) consists of complex group of tumors that develop predominantly in the digestive or respiratory tracts, but can occur in many areas of the body. These tumors arise from cells called neuroendocrine cells. Like all cancers, NETs develop when the specialized cells undergo changes causing the...
Find More
Axovant rely on new gene therapy, Rise in Shares Roivant Neuro Company has licensed Oxford Biomedica’s gene therapy OXB-102, now AXO-Lenti-PD, for Parkinson’s disease (PD). Spark Therapeutics’ Former CTO Fraser Wright, Ph.D has been hired as its new CTO. Axovant expects to stimulate a phase 1/2 dose escalation stu...
Find More
Immune checkpoints are the regulators of immune activation. They play a key role in maintaining immune homeostasis and preventing autoimmunity. Immune checkpoint activators are small molecules responsible for maintenance, modulation, and regulation of immune responses. Its significance in immune-therapeutics is back...
Find More
Systemic Lupus Erythematosus (SLE) is a chronic, multisystem, inflammatory autoimmune disease which leads to weakening of the immune system. It results in systemic inflammation which affects multiple organs such as kidneys, the tissue lining the lungs (pleura), heart (pericardium), and brain. Acute Cutaneous Lupus, ...
Find More
KINECT 4 Phase III Study Demonstrating INGREZZA® Improves Tardive Dyskinesia Symptoms Neurocrine Biosciences, Inc. announced new long-term data from the KINECT 4 Phase III open-label study demonstrate that once-daily INGREZZA® (valbenazine) capsules, the first FDA approved treatment for adults with tardive dyskinesi...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.